The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: a promising therapeutic target
- PMID: 38419070
- PMCID: PMC10903003
- DOI: 10.1186/s12935-024-03221-8
The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: a promising therapeutic target
Abstract
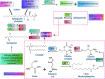
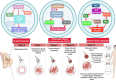
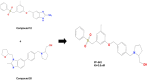
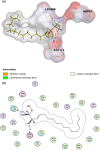
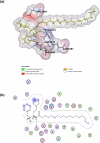
Cancer chemoresistance is a problematic dilemma that significantly restrains numerous cancer management protocols. It can promote cancer recurrence, spreading of cancer, and finally, mortality. Accordingly, enhancing the responsiveness of cancer cells towards chemotherapies could be a vital approach to overcoming cancer chemoresistance. Tumour cells express a high level of sphingosine kinase-1 (SphK1), which acts as a protooncogenic factor and is responsible for the synthesis of sphingosine-1 phosphate (S1P). S1P is released through a Human ATP-binding cassette (ABC) transporter to interact with other phosphosphingolipids components in the interstitial fluid in the tumor microenvironment (TME), provoking communication, progression, invasion, and tumor metastasis. Also, S1P is associated with several impacts, including anti-apoptotic behavior, metastasis, mesenchymal transition (EMT), angiogenesis, and chemotherapy resistance. Recent reports addressed high levels of S1P in several carcinomas, including ovarian, prostate, colorectal, breast, and HCC. Therefore, targeting the S1P/SphK signaling pathway is an emerging therapeutic approach to efficiently attenuate chemoresistance. In this review, we comprehensively discussed S1P functions, metabolism, transport, and signaling. Also, through a bioinformatic framework, we pointed out the alterations of SphK1 gene expression within different cancers with their impact on patient survival, and we demonstrated the protein-protein network of SphK1, elaborating its sparse roles. Furthermore, we made emphasis on different machineries of cancer resistance and the tight link with S1P. We evaluated all publicly available SphK1 inhibitors and their inhibition activity using molecular docking and how SphK1 inhibitors reduce the production of S1P and might reduce chemoresistance, an approach that might be vital in the course of cancer treatment and prognosis.
Keywords: Cancer; Chemoresistance; Recurrence; S1P; SphK1; TME.
© 2024. The Author(s).
Conflict of interest statement
All authors consent to publication. The authors declare that they have no competing interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



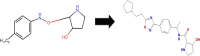


Similar articles
-
Sphingosine kinase 1/sphingosine-1-phosphate (S1P)/S1P receptor axis is involved in ovarian cancer angiogenesis.Oncotarget. 2017 Aug 24;8(43):74947-74961. doi: 10.18632/oncotarget.20471. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088837 Free PMC article.
-
Triple Negative Breast Cancer Depends on Sphingosine Kinase 1 (SphK1)/Sphingosine-1-Phosphate (S1P)/Sphingosine 1-Phosphate Receptor 3 (S1PR3)/Notch Signaling for Metastasis.Med Sci Monit. 2018 Apr 1;24:1912-1923. doi: 10.12659/msm.905833. Med Sci Monit. 2018. PMID: 29605826 Free PMC article.
-
Interaction of microRNAs with sphingosine kinases, sphingosine-1 phosphate, and sphingosine-1 phosphate receptors in cancer.Discov Oncol. 2021 Sep 20;12(1):33. doi: 10.1007/s12672-021-00430-9. Discov Oncol. 2021. PMID: 35201458 Free PMC article. Review.
-
AP-1 regulates sphingosine kinase 1 expression in a positive feedback manner in glomerular mesangial cells exposed to high glucose.Cell Signal. 2014 Mar;26(3):629-38. doi: 10.1016/j.cellsig.2013.12.002. Epub 2013 Dec 14. Cell Signal. 2014. PMID: 24342046
-
S1P Signaling in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1223:129-153. doi: 10.1007/978-3-030-35582-1_7. Adv Exp Med Biol. 2020. PMID: 32030688 Review.
Cited by
-
Drug Resistance: The Role of Sphingolipid Metabolism.Int J Mol Sci. 2025 Apr 15;26(8):3716. doi: 10.3390/ijms26083716. Int J Mol Sci. 2025. PMID: 40332322 Free PMC article. Review.
-
The Combination of PF-543 and TRAIL Effectively Induces Apoptotic Cell Death and Inhibits Stem Cell-Like Properties Through the SPHK1/S1PR1/STAT3 Pathway in TRAIL-Resistant Colorectal Cancer Cells.Dig Dis Sci. 2025 Sep;70(9):3041-3055. doi: 10.1007/s10620-025-09091-y. Epub 2025 Jun 5. Dig Dis Sci. 2025. PMID: 40467915
-
Medicinal plants: nutritional, immunological and therapeutic role in treating cancer-related malnutrition: a comprehensive review.Cancer Cell Int. 2025 Jul 16;25(1):266. doi: 10.1186/s12935-025-03720-2. Cancer Cell Int. 2025. PMID: 40671046 Free PMC article. Review.
-
Identification of Sphingosine Kinase 1 as a Novel Protein Regulated by High Molecular Weight Hyaluronan in Ovarian Cancer.J Cell Mol Med. 2025 May;29(9):e70574. doi: 10.1111/jcmm.70574. J Cell Mol Med. 2025. PMID: 40356021 Free PMC article.
-
Concomitant targeting of FLT3 and SPHK1 exerts synergistic cytotoxicity in FLT3-ITD+ acute myeloid leukemia by inhibiting β-catenin activity via the PP2A-GSK3β axis.Cell Commun Signal. 2024 Aug 7;22(1):391. doi: 10.1186/s12964-024-01774-9. Cell Commun Signal. 2024. PMID: 39113090 Free PMC article.
References
-
- Alkafaas SS, Diab T, Shalaby T, Hessien M. Dexamethasone improves the responsiveness of hepatoma cells for both free and solvent containing paclitaxel in vitro. Egypt J Biochem Mol Biol. 2019;37:110.
-
- Elsalahaty MI, Alkafaas SS, Bashir AO, El-Tarabily KA, El-Saadony MT, Yousef EH. Revealing the Association Between Vitamin D Metabolic Pathway Gene Variants and Lung Cancer Risk: A Systematic Review and Meta-Analysis. Front in Genet. 2024;15:1302527. doi: 10.3389/fgene.2024.1302527. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

