An e-mental health intervention to reduce depression symptoms in individuals with obesity: study protocol for the randomized, controlled, two-armed, confirmatory LightMood trial
- PMID: 38419096
- PMCID: PMC10900592
- DOI: 10.1186/s13063-024-07970-9
An e-mental health intervention to reduce depression symptoms in individuals with obesity: study protocol for the randomized, controlled, two-armed, confirmatory LightMood trial
Abstract
Background: Patients with obesity often experience psychological distress, specifically depression symptoms. Due to various barriers, such as limitations of healthcare offers, digital interventions, for example medical apps, can provide a suitable approach to support affected people. In the envisaged prospective randomized controlled trial, we aim to examine the efficacy of the LightMood intervention. The LightMood intervention is a manualized and user-centered, digital intervention for patients with obesity, with a duration of 4 months, which contains elements of cognitive behavioral therapy and mindfulness-based and skills-based exercises. We expect the LightMood intervention to be superior to treatment as usual (TAU) in terms of reducing depression symptoms.
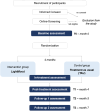
Methods: The trial incorporates four distinct measurement time points: the baseline assessment, the post-treatment assessment, and 1- and 3-month follow-up assessments. Furthermore, we implemented in-treatment assessments for both groups. Participants will be randomized into two groups (LightMood intervention vs TAU). The aim is to include 128 participants (64 per group) in the study. Inclusion criteria are patients who are obese, at least 18 years old, with a private Internet access, and with adequate digital literacy and show depression symptoms (PHQ ≥ 10). Exclusion criteria are weekly outpatient individual psychotherapy, obesity surgery within the last year or planned within the next 7 months, no private Internet access, and the prescription of a new psychotropic drug within the last 2 weeks. The primary outcome is the post-assessment reduction in depression symptoms. Secondary outcomes will include the improvement in self-efficacy, quality of life, mindfulness, reduction in eating disorder symptoms, and body mass index (BMI). Furthermore, we expect a positive development of depression symptoms throughout the different time points (T1, T2, and T3) in patients with obesity.
Discussion: LightMood is an evidence-based, efficient, low-threshold online intervention that aims to reduce depression symptoms in people with obesity. Online interventions could offer a promising alternative to conventional face-to-face therapy. The primary objective of the current study is to add essential insight into the feasibility, efficacy, effectiveness, and acceptance of e-mental health interventions for people with obesity and depression symptoms.
Trial registration: German Clinical Trial Register (DRKS), DRKS00029219. Registered on May 19, 2023.
Keywords: CBT; Depression; Mindfulness; Obesity; Online-intervention; Randomized controlled trial; eHealth.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
-
Web-based mindfulness and skills-based distress reduction for patients with cancer: study protocol of the multicentre, randomised, controlled confirmatory intervention trial Reduct.BMJ Open. 2022 Jun 1;12(6):e056973. doi: 10.1136/bmjopen-2021-056973. BMJ Open. 2022. PMID: 35649607 Free PMC article.
-
Study protocol of a multicenter randomized controlled trial comparing the effectiveness of group and individual internet-based Mindfulness-Based Cognitive Therapy with treatment as usual in reducing psychological distress in cancer patients: the BeMind study.BMC Psychol. 2015 Aug 13;3(1):27. doi: 10.1186/s40359-015-0084-1. eCollection 2015. BMC Psychol. 2015. PMID: 26273472 Free PMC article. Clinical Trial.
-
A self-guided Internet-based intervention for individuals with chronic pain and depressive symptoms: study protocol of a randomized controlled trial.Trials. 2023 Jul 11;24(1):453. doi: 10.1186/s13063-023-07440-8. Trials. 2023. PMID: 37434163 Free PMC article.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
-
Telephone interventions for symptom management in adults with cancer.Cochrane Database Syst Rev. 2020 Jun 2;6(6):CD007568. doi: 10.1002/14651858.CD007568.pub2. Cochrane Database Syst Rev. 2020. PMID: 32483832 Free PMC article.
Cited by
-
What internet- and mobile-based interventions are currently available for adults with overweight or obesity experiencing symptoms of depression? A systematic review.Int J Obes (Lond). 2025 Jan;49(1):63-75. doi: 10.1038/s41366-024-01654-9. Epub 2024 Oct 21. Int J Obes (Lond). 2025. PMID: 39433892 Free PMC article.
References
-
- World Health Organization. Obesity and overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed on 3 Jan 2023.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical