A novel extracellular vesicles production system harnessing matrix homeostasis and macrophage reprogramming mitigates osteoarthritis
- PMID: 38419097
- PMCID: PMC10903078
- DOI: 10.1186/s12951-024-02324-8
A novel extracellular vesicles production system harnessing matrix homeostasis and macrophage reprogramming mitigates osteoarthritis
Abstract
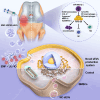
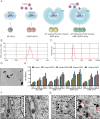
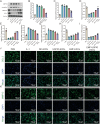
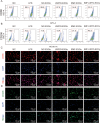
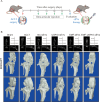
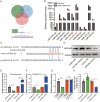
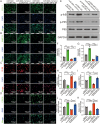
Osteoarthritis (OA) is a degenerative disease that significantly impairs quality of life. There is a pressing need for innovative OA therapies. While small extracellular vesicles (sEVs) show promising therapeutic effects against OA, their limited yield restricts clinical translation. Here, we devised a novel production system for sEVs that enhances both their yield and therapeutic properties. By stimulating mesenchymal stem cells (MSCs) using electromagnetic field (EMF) combined with ultrasmall superparamagnetic iron oxide (USPIO) particles, we procured an augmented yield of EMF-USPIO-sEVs. These vesicles not only activate anabolic pathways but also inhibit catabolic activities, and crucially, they promote M2 macrophage polarization, aiding cartilage regeneration. In an OA mouse model triggered by anterior cruciate ligament transection surgery, EMF-USPIO-sEVs reduced OA severity, and augmented matrix synthesis. Moreover, they decelerated OA progression through the microRNA-99b/MFG-E8/NF-κB signaling axis. Consequently, EMF-USPIO-sEVs present a potential therapeutic option for OA, acting by modulating matrix homeostasis and macrophage polarization.
Keywords: Electromagnetic field; Extracellular vesicles; Macrophage; Matrix homeostasis; Osteoarthritis; Reprogramming.
© 2024. The Author(s).
Conflict of interest statement
The authors have no financial disclosures or conflicts of interest with the research presented.
Figures



Similar articles
-
Comparison of Curative Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells and Their Small Extracellular Vesicles in Treating Osteoarthritis.Int J Nanomedicine. 2021 Dec 16;16:8185-8202. doi: 10.2147/IJN.S336062. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34938076 Free PMC article.
-
M2 Macrophage-Derived Extracellular Vesicles Encapsulated in Hyaluronic Acid Alleviate Osteoarthritis by Modulating Macrophage Polarization.ACS Biomater Sci Eng. 2024 May 13;10(5):3355-3377. doi: 10.1021/acsbiomaterials.3c01833. Epub 2024 Apr 2. ACS Biomater Sci Eng. 2024. PMID: 38563817
-
Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages.J Nanobiotechnology. 2022 Jan 20;20(1):38. doi: 10.1186/s12951-021-01236-1. J Nanobiotechnology. 2022. PMID: 35057811 Free PMC article.
-
New mesenchymal stem/stromal cell-based strategies for osteoarthritis treatment: targeting macrophage-mediated inflammation to restore joint homeostasis.J Mol Med (Berl). 2025 Jun;103(6):651-669. doi: 10.1007/s00109-025-02547-8. Epub 2025 Apr 24. J Mol Med (Berl). 2025. PMID: 40272537 Review.
-
MSC-EVs alleviate osteoarthritis by regulating microenvironmental cells in the articular cavity and maintaining cartilage matrix homeostasis.Ageing Res Rev. 2023 Mar;85:101864. doi: 10.1016/j.arr.2023.101864. Epub 2023 Jan 24. Ageing Res Rev. 2023. PMID: 36707035 Review.
Cited by
-
Multi-omics analysis of small extracellular vesicles in osteoarthritis: bridging the gap between molecular insights and clinical applications.Burns Trauma. 2025 Mar 20;13:tkaf023. doi: 10.1093/burnst/tkaf023. eCollection 2025. Burns Trauma. 2025. PMID: 40740687 Free PMC article. Review.
-
Mesenchymal stromal cells-derived extracellular vesicles in cartilage regeneration: potential and limitations.Stem Cell Res Ther. 2025 Jan 23;16(1):11. doi: 10.1186/s13287-025-04135-6. Stem Cell Res Ther. 2025. PMID: 39849578 Free PMC article.
-
Targeting osteoarthritis with small extracellular vesicle therapy: potential and perspectives.Front Bioeng Biotechnol. 2025 Jun 20;13:1570526. doi: 10.3389/fbioe.2025.1570526. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40621210 Free PMC article.
-
Energizing Healing with Electromagnetic Field Therapy in Musculoskeletal Disorders.J Orthop Sports Med. 2024;6(2):89-106. doi: 10.26502/josm.511500147. Epub 2024 May 17. J Orthop Sports Med. 2024. PMID: 39036742 Free PMC article.
References
-
- Anonymous Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet. 2018;392:1789–858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

