Scleritis following intravitreal brolucizumab injection: a case series
- PMID: 38419100
- PMCID: PMC10902930
- DOI: 10.1186/s13256-024-04402-9
Scleritis following intravitreal brolucizumab injection: a case series
Abstract
Background: This study reports the first cases of scleritis following intravitreal brolucizumab (IVBr) injection for nAMD, emphasizing the need to be aware of the possibility of scleritis following IVBr injections.
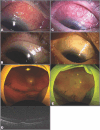
Case presentation: Case 1. A 74-year-old Japanese man with nAMD complained of conjunctivitis and decreased vision in the right eye 8 days after his eighth IVBr injection. Examination revealed scleritis without anterior inflammation. Topical 0.1% betamethasone and 0.3% gatifloxacin eye drops were started. The scleritis worsened in the following 2 weeks and became painful. He underwent sub-Tenon's capsule triamcinolone acetonide (STTA) injection. Two days later, he returned with a complaint of severe vision loss. Fundus examination revealed retinal artery occlusion, vasculitis, and vitreous opacity in the right eye. Vitreous surgery was performed.
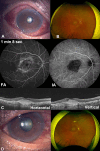
Case 2: An 85-year-old Japanese woman with nAMD in the right eye complained of reddening of the eye 27 days after her fifth IVBr injection. Examination showed conjunctivitis and scleritis without anterior inflammation in the right eye. She was started on 0.1% fluorometholone and 0.5% levofloxacin hydrate eye drops. The scleritis worsened in the following 3 weeks. Her treatment was switched to 0.1% betamethasone eye drops. One month later, the scleritis had improved and a sixth IVBr injection was administered. There was no worsening of the scleritis at that time. However, 1 month after a seventh IVBr injection, she complained of severe hyperemia and decreased vision. Fundus examination revealed vitreous opacification. She underwent STTA, and the vitreous opacity improved in 24 days. Case 3. A 57-year-old Japanese man with nAMD complained of pain and decreased vision in the right eye 21 days after a fourth IVBr injection. Examination revealed scleritis with high intraocular pressure but no anterior chamber or fundus inflammation. STTA and topical eye drops were performed. One month later, scleritis improved but visual acuity didn't due to progression of nAMD.
Conclusions: Intraocular inflammation following IVBr injection may progress to the posterior segment. Scleritis can occur after IVBr injection, and topical eye drops alone may not be sufficient for initial treatment. Clinicians should consider the possibility of scleritis in patients with worsening inflammation after IVBr injection.
Keywords: Brolucizumab; Case report; Intraocular inflammation; Scleritis.
© 2024. The Author(s).
Conflict of interest statement
The following authors have no financial disclosures: T.T., R.T., H.Y., Y.K., R.T. S.I.: Lecturer’s fees from Kowa and Novartis and grants from Novartis, outside this work. H.T.: Patent pending of this work; lecturer’s fees from Santen, Kowa, Senju, Novartis, and Bayer; grants from Senju, Novartis, and Bayer; a founder of DeepEyeVision, Inc.; patents outside this work. K.N: Lecturer’s fees from Novartis outside this work. Y.A.: Lecturer’s fees from Santen, Kowa, Senju, and Bayer; grants from Heiwa-Iyo, Novartis and Bayer outside this work. H.K.: Lecturer’s fees from Otsuka, Senju, Mitsubishi-Tanabe, Kowa, Santen, Novartis, and Zeiss; grants from Senju, Linical, DeepEyeVision, HOYA, Santen, Heiwa-Iyou, and Bayer, outside this work.
Figures

Similar articles
-
Retinal arterial occlusive vasculitis after multiple intravitreal brolucizumab injections for diabetic macular edema.Am J Ophthalmol Case Rep. 2022 Dec 30;29:101788. doi: 10.1016/j.ajoc.2022.101788. eCollection 2023 Mar. Am J Ophthalmol Case Rep. 2022. PMID: 36632338 Free PMC article.
-
Occlusive retinal vasculitis and scleritis following brolucizumab treatment: A case report.Medicine (Baltimore). 2024 Oct 18;103(42):e40154. doi: 10.1097/MD.0000000000040154. Medicine (Baltimore). 2024. PMID: 39432659 Free PMC article.
-
Scedosporium apiospermum infectious scleritis following posterior subtenon triamcinolone acetonide injection: a case report and literature review.BMC Ophthalmol. 2018 Feb 13;18(1):40. doi: 10.1186/s12886-018-0707-4. BMC Ophthalmol. 2018. PMID: 29433463 Free PMC article. Review.
-
Intraocular Inflammation Secondary to Intravitreal Brolucizumab Injection for Neovascular Age-Related Macular Degeneration in a Patient with Cognitive Impairment.Medicina (Kaunas). 2023 Oct 19;59(10):1856. doi: 10.3390/medicina59101856. Medicina (Kaunas). 2023. PMID: 37893574 Free PMC article.
-
Fungal Necrotizing Scleritis After Intravitreal Injection Therapy.Cornea. 2021 Dec 1;40(12):1617-1619. doi: 10.1097/ICO.0000000000002670. Cornea. 2021. PMID: 34749383 Review.
References
-
- Matsumoto H, Hoshino J, Mukai R, Nakamura K, Akiyama H. Short-term outcomes of intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 choroidal neovascularization including polypoidal choroidal vasculopathy. Sci Rep. 2021;11(1):6759. doi: 10.1038/s41598-021-86014-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

