Prevention of post-operative delirium using an overnight infusion of dexmedetomidine in patients undergoing cardiac surgery: a pragmatic, randomized, double-blind, placebo-controlled trial
- PMID: 38419119
- PMCID: PMC10902989
- DOI: 10.1186/s13054-024-04842-1
Prevention of post-operative delirium using an overnight infusion of dexmedetomidine in patients undergoing cardiac surgery: a pragmatic, randomized, double-blind, placebo-controlled trial
Abstract
Background: After cardiac surgery, post-operative delirium (PoD) is acknowledged to have a significant negative impact on patient outcome. To date, there is no valuable and specific treatment for PoD. Critically ill patients often suffer from poor sleep condition. There is an association between delirium and sleep quality after cardiac surgery. This study aimed to establish whether promoting sleep using an overnight infusion of dexmedetomidine reduces the incidence of delirium after cardiac surgery.
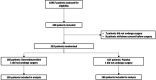
Methods: Randomized, pragmatic, multicentre, double-blind, placebo controlled trial from January 2019 to July 2021. All adult patients aged 65 years or older requiring elective cardiac surgery were randomly assigned 1:1 either to the dexmedetomidine group or the placebo group on the day of surgery. Dexmedetomidine or matched placebo infusion was started the night after surgery from 8 pm to 8 am and administered every night while the patient remained in ICU, or for a maximum of 7 days. Primary outcome was the occurrence of postoperative delirium (PoD) within the 7 days after surgery.
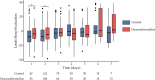
Results: A total of 348 patients provided informed consent, of whom 333 were randomized: 331 patients underwent surgery and were analysed (165 assigned to dexmedetomidine and 166 assigned to placebo). The incidence of PoD was not significantly different between the two groups (12.6% vs. 12.4%, p = 0.97). Patients treated with dexmedetomidine had significantly more hypotensive events (7.3% vs 0.6%; p < 0.01). At 3 months, functional outcomes (Short-form 36, Cognitive failure questionnaire, PCL-5) were comparable between the two groups.
Conclusion: In patients recovering from an elective cardiac surgery, an overnight infusion of dexmedetomidine did not decrease postoperative delirium. Trial registration This trial was registered on ClinicalTrials.gov (number: NCT03477344; date: 26th March 2018).
Keywords: Cardiac surgery; Delirium; Dexmedetomidine; Sleep quality.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest for this manuscript.
Figures
References
-
- Hughes CG, Hayhurst CJ, Pandharipande PP, Shotwell MS, Feng X, Wilson JE, Brummel NE, Girard TD, Jackson JC, Ely EW, Patel MB. Association of delirium during critical illness with mortality: multicenter prospective cohort study. Anesth Analg. 2021;133(5):1152–1161. doi: 10.1213/ANE.0000000000005544. - DOI - PMC - PubMed
-
- Girard TD, Thompson JL, Pandharipande PP, Brummel NE, Jackson JC, Patel MB, Hughes CG, Chandrasekhar R, Pun BT, Boehm LM, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. doi: 10.1016/S2213-2600(18)30062-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

