Effect of deletion of the protein kinase PRKD1 on development of the mouse embryonic heart
- PMID: 38419169
- PMCID: PMC11161829
- DOI: 10.1111/joa.14033
Effect of deletion of the protein kinase PRKD1 on development of the mouse embryonic heart
Abstract
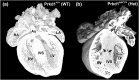
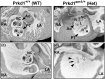
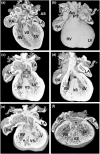
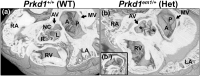
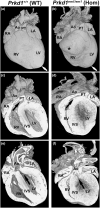
Congenital heart disease (CHD) is the most common congenital anomaly, with an overall incidence of approximately 1% in the United Kingdom. Exome sequencing in large CHD cohorts has been performed to provide insights into the genetic aetiology of CHD. This includes a study of 1891 probands by our group in collaboration with others, which identified three novel genes-CDK13, PRKD1, and CHD4, in patients with syndromic CHD. PRKD1 encodes a serine/threonine protein kinase, which is important in a variety of fundamental cellular functions. Individuals with a heterozygous mutation in PRKD1 may have facial dysmorphism, ectodermal dysplasia and may have CHDs such as pulmonary stenosis, atrioventricular septal defects, coarctation of the aorta and bicuspid aortic valve. To obtain a greater appreciation for the role that this essential protein kinase plays in cardiogenesis and CHD, we have analysed a Prkd1 transgenic mouse model (Prkd1em1) carrying deletion of exon 2, causing loss of function. High-resolution episcopic microscopy affords detailed morphological 3D analysis of the developing heart and provides evidence for an essential role of Prkd1 in both normal cardiac development and CHD. We show that homozygous deletion of Prkd1 is associated with complex forms of CHD such as atrioventricular septal defects, and bicuspid aortic and pulmonary valves, and is lethal. Even in heterozygotes, cardiac differences occur. However, given that 97% of Prkd1 heterozygous mice display normal heart development, it is likely that one normal allele is sufficient, with the defects seen most likely to represent sporadic events. Moreover, mRNA and protein expression levels were investigated by RT-qPCR and western immunoblotting, respectively. A significant reduction in Prkd1 mRNA levels was seen in homozygotes, but not heterozygotes, compared to WT littermates. While a trend towards lower PRKD1 protein expression was seen in the heterozygotes, the difference was only significant in the homozygotes. There was no compensation by the related Prkd2 and Prkd3 at transcript level, as evidenced by RT-qPCR. Overall, we demonstrate a vital role of Prkd1 in heart development and the aetiology of CHD.
Keywords: Prkd1; congenital heart disease; high‐resolution episcopic microscopy; protein kinase; protein kinase D1.
© 2024 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures

Similar articles
-
Cyclin-dependent kinase 13 is indispensable for normal mouse heart development.J Anat. 2025 Apr;246(4):616-630. doi: 10.1111/joa.14175. Epub 2024 Nov 18. J Anat. 2025. PMID: 39556044 Free PMC article.
-
Novel Autosomal Recessive Splice-Altering Variant in PRKD1 Is Associated with Congenital Heart Disease.Genes (Basel). 2021 Apr 21;12(5):612. doi: 10.3390/genes12050612. Genes (Basel). 2021. PMID: 33919081 Free PMC article.
-
Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing.Nat Genet. 2016 Sep;48(9):1060-5. doi: 10.1038/ng.3627. Epub 2016 Aug 1. Nat Genet. 2016. PMID: 27479907 Free PMC article.
-
PRKD1-related telangiectasia-ectodermal dysplasia-brachydactyly-cardiac anomaly syndrome: Case report and review of the literature.Eur J Med Genet. 2024 Jun;69:104942. doi: 10.1016/j.ejmg.2024.104942. Epub 2024 Apr 25. Eur J Med Genet. 2024. PMID: 38677542 Review.
-
Increased intracranial pressure in a patient with Congenital Heart Defect and Ectodermal Dysplasia (CHDED): Extension of phenotype and review of literature.Am J Med Genet A. 2023 Feb;191(2):554-558. doi: 10.1002/ajmg.a.63023. Epub 2022 Oct 29. Am J Med Genet A. 2023. PMID: 36308391 Review.
Cited by
-
Molecular convergence of risk variants for congenital heart defects leveraging a regulatory map of the human fetal heart.medRxiv [Preprint]. 2024 Nov 22:2024.11.20.24317557. doi: 10.1101/2024.11.20.24317557. medRxiv. 2024. PMID: 39606363 Free PMC article. Preprint.
-
Cyclin-dependent kinase 13 is indispensable for normal mouse heart development.J Anat. 2025 Apr;246(4):616-630. doi: 10.1111/joa.14175. Epub 2024 Nov 18. J Anat. 2025. PMID: 39556044 Free PMC article.
References
-
- Alter, S. , Zimmer, A.D. , Park, M. , Gong, J. , Caliebe, A. , Fölster‐Holst, R. et al. (2021) Telangiectasia‐ectodermal dysplasia‐brachydactyly‐cardiac anomaly syndrome is caused by de novo mutations in protein kinase D1. Journal of Medical Genetics, 58, 415–421. - PubMed
-
- Anderson, R.H. , Mohun, T.J. , Spicer, D.E. , Bamforth, S.D. , Brown, N.A. , Chaudhry, B. et al. (2014) Myths and realities relating to development of the arterial valves. Journal of Cardiovascular Development and Disease, 1, 177–200.
-
- Anderson, R.H. , Webb, S. & Brown, N.A. (1998) The mouse with trisomy 16 as a model of human hearts with common atrioventricular junction. Cardiovascular Research, 39, 155–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

