Comparison between tofacitinib and ustekinumab as a third-line therapy in refractory ulcerative colitis: A multicenter international study
- PMID: 38419274
- PMCID: PMC11176899
- DOI: 10.1002/ueg2.12492
Comparison between tofacitinib and ustekinumab as a third-line therapy in refractory ulcerative colitis: A multicenter international study
Abstract
Background: Ustekinumab and tofacitinib have recently been approved for the management of moderate to severe ulcerative colitis (UC). However, there is no evidence on how they should be positioned in the therapeutic algorithm. The aim of this study was to compare tofacitinib and ustekinumab as third-line therapies in UC patients in whom anti-TNF and vedolizumab had failed.
Methods: This was a multicenter retrospective observational study. The primary outcome was disease progression, defined as the need for steroids, therapy escalation, UC-related hospitalization and/or surgery. Secondary outcomes were clinical remission, normalization of C-reactive protein, endoscopic remission, treatment withdrawal, and adverse events.
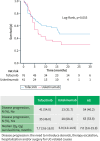
Results: One-hundred seventeen UC patients were included in the study and followed for a median time of 11.6 months (q1-q3, 5.5-18.7). Overall, 65% of patients were treated with tofacitinib and 35% with ustekinumab. In the entire study cohort, 63 patients (54%) had disease progression during the follow-up period. Treatment with ustekinumab predicted increased risk of disease progression compared to treatment with tofacitinib in Cox regression analysis (HR: 1.93 [95% CI: 1.06-3.50] p = 0.030). Twenty-eight (68%) patients in the ustekinumab group and 35 (46%) in the tofacitinib group had disease progression over the follow-up period (log-rank test, p < 0.054). No significant differences were observed for the secondary outcomes. Six and 22 adverse events occurred in the ustekinumab and tofacitinib groups, respectively (15% vs. 31%, p = 0.11).
Conclusions: Tofacitinib was more efficacious in reducing disease progression than ustekinumab in this cohort of refractory UC patients. However, prospective head-to-head clinical trials are needed as to confirm these data.
Keywords: biologics; inflammatory bowel disease; tofacitinib.
© 2023 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC. on behalf of United European Gastroenterology.
Conflict of interest statement
Allocca M received consulting fees from Nikkiso Europe, Mundipharma, Janssen, Abbvie, Ferring, Galapagos and Pfizer; Peyrin‐Biroulet L reported personal fees from Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger‐Ingelheim, Lilly, HAC‐Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, and Samsung Biosepsis; Ellul P received consulting fees from Janssen; Fiorino G received consultancy fees from Ferring, AbbVie, Takeda, Janssen, Amgen, Sandoz, Celltrion, Galapagos; D’Amico F has served as a speaker for Janssen, Galapagos, Sandoz, and Omega Pharma; he has also served as advisory board member for Abbvie, Galapagos, and Nestlè; Danese S served as a speaker, consultant and advisory board member for Schering‐Plough, Abbott (AbbVie) Laboratories, Merck, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alfa Wasserman, Genentech, Grunenthal, Pfizer, AstraZeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor and Johnson and Johnson; Gisbert JP has served as speaker, consultant, and advisory member for or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine and Vifor Pharma; Konstantinos Karmiris has served as speaker, consultant and advisory board member for Abbvie, Amgen, Ferring, Galenica, Genesis, Janssen, MSD, Pfizer, Takeda and Vianex; María Chaparro: Speaker, consultant or research or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Biogen, Gilead and Lilly; All the other authors did not have any conflict of interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials