The CK1ε/SIAH1 axis regulates AXIN1 stability in colorectal cancer cells
- PMID: 38419282
- PMCID: PMC11467792
- DOI: 10.1002/1878-0261.13624
The CK1ε/SIAH1 axis regulates AXIN1 stability in colorectal cancer cells
Abstract
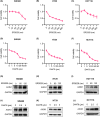
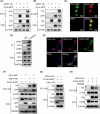
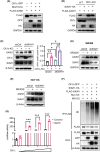
Casein kinase 1ε (CK1ε) and axis inhibitor 1 (AXIN1) are crucial components of the β-catenin destruction complex in canonical Wnt signaling. CK1ε has been shown to interact with AXIN1, but its physiological function and role in tumorigenesis remain unknown. In this study, we found that CK1δ/ε inhibitors significantly enhanced AXIN1 protein level in colorectal cancer (CRC) cells through targeting CK1ε. Mechanistically, CK1ε promoted AXIN1 degradation by the ubiquitin-proteasome pathway by promoting the interaction of E3 ubiquitin-protein ligase SIAH1 with AXIN1. Genetic or pharmacological inhibition of CK1ε and knockdown of SIAH1 downregulated the expression of Wnt/β-catenin-dependent genes, suppressed the viability of CRC cells, and restrained tumorigenesis and progression of CRC in vitro and in vivo. In summary, our results demonstrate that CK1ε exerted its oncogenic role in CRC occurrence and progression by regulating the stability of AXIN1. These findings reveal a novel mechanism by which CK1ε regulates the Wnt/β-catenin signaling pathway and highlight the therapeutic potential of targeting the CK1ε/SIAH1 axis in CRC.
Keywords: AXIN1; CK1ε; SIAH1; Wnt/β‐catenin; colorectal cancer; ubiquitination.
© 2024 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
The E3 ubiquitin ligase RNF146 promotes colorectal cancer by activating the Wnt/β-catenin pathway via ubiquitination of Axin1.Biochem Biophys Res Commun. 2018 Sep 5;503(2):991-997. doi: 10.1016/j.bbrc.2018.06.107. Epub 2018 Jun 25. Biochem Biophys Res Commun. 2018. PMID: 29932918
-
R-spondin-1 induces Axin degradation via the LRP6-CK1ε axis.Cell Commun Signal. 2024 Jan 5;22(1):14. doi: 10.1186/s12964-023-01456-y. Cell Commun Signal. 2024. PMID: 38183076 Free PMC article.
-
USP44 suppresses proliferation and enhances apoptosis in colorectal cancer cells by inactivating the Wnt/β-catenin pathway via Axin1 deubiquitination.Cell Biol Int. 2020 Aug;44(8):1651-1659. doi: 10.1002/cbin.11358. Epub 2020 Apr 21. Cell Biol Int. 2020. PMID: 32285989 Free PMC article.
-
Casein kinase 1 epsilon (CK1ε) as a potential therapeutic target in chronic liver disease.J Vet Sci. 2025 May;26(3):e30. doi: 10.4142/jvs.24321. J Vet Sci. 2025. PMID: 40461423 Free PMC article. Review.
-
TRIM11 promotes lymphomas by activating the β-catenin signaling and Axin1 ubiquitination degradation.Exp Cell Res. 2020 Feb 15;387(2):111750. doi: 10.1016/j.yexcr.2019.111750. Epub 2019 Nov 28. Exp Cell Res. 2020. PMID: 31786079 Review.
Cited by
-
The emerging role of E3 ubiquitin ligases and deubiquitinases in metabolic dysfunction-associated steatotic liver disease.J Transl Med. 2025 Mar 25;23(1):368. doi: 10.1186/s12967-025-06255-2. J Transl Med. 2025. PMID: 40133964 Free PMC article. Review.
-
Ubiquitin modification in the regulation of tumor immunotherapy resistance mechanisms and potential therapeutic targets.Exp Hematol Oncol. 2024 Aug 30;13(1):91. doi: 10.1186/s40164-024-00552-0. Exp Hematol Oncol. 2024. PMID: 39223632 Free PMC article. Review.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Schloss J, Ryan K, Steel A. Corrigendum to a randomised, double‐blind, placebo‐controlled clinical trial found that a novel herbal formula UROX(R) BEDTIME BUDDY assisted children for the treatment of nocturnal enuresis. Phytomedicine. 2022;99:153992. - PubMed
MeSH terms
Substances
Grants and funding
- JCYJ20210324094405014/Shenzhen Science and Technology Innovation Program
- JCYJ20200109105001821/Shenzhen Key Basic Research Program
- 2020A1515010340/Natural Science Foundation of Guangdong Province
- 2020A1515010543/Natural Science Foundation of Guangdong Province
- 2021A1515011164/Natural Science Foundation of Guangdong Province
- 2022A1515010598/Natural Science Foundation of Guangdong Province
- 31900881/National Natural Science Foundation of China
- 31970739/National Natural Science Foundation of China
- 32100700/National Natural Science Foundation of China
- 81802662/National Natural Science Foundation of China
- 82273485/National Natural Science Foundation of China
- U21A20421/National Natural Science Foundation of China
- 20200826134656001/Stable Support Project of Shenzhen
- 20231120113237002/Stable Support Project of Shenzhen
LinkOut - more resources
Full Text Sources
Medical

