ATF4-dependent increase in mitochondrial-endoplasmic reticulum tethering following OPA1 deletion in skeletal muscle
- PMID: 38419397
- PMCID: PMC11144302
- DOI: 10.1002/jcp.31204
ATF4-dependent increase in mitochondrial-endoplasmic reticulum tethering following OPA1 deletion in skeletal muscle
Abstract
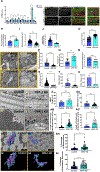
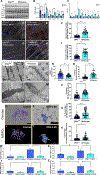
Mitochondria and endoplasmic reticulum (ER) contact sites (MERCs) are protein- and lipid-enriched hubs that mediate interorganellar communication by contributing to the dynamic transfer of Ca2+, lipid, and other metabolites between these organelles. Defective MERCs are associated with cellular oxidative stress, neurodegenerative disease, and cardiac and skeletal muscle pathology via mechanisms that are poorly understood. We previously demonstrated that skeletal muscle-specific knockdown (KD) of the mitochondrial fusion mediator optic atrophy 1 (OPA1) induced ER stress and correlated with an induction of Mitofusin-2, a known MERC protein. In the present study, we tested the hypothesis that Opa1 downregulation in skeletal muscle cells alters MERC formation by evaluating multiple myocyte systems, including from mice and Drosophila, and in primary myotubes. Our results revealed that OPA1 deficiency induced tighter and more frequent MERCs in concert with a greater abundance of MERC proteins involved in calcium exchange. Additionally, loss of OPA1 increased the expression of activating transcription factor 4 (ATF4), an integrated stress response (ISR) pathway effector. Reducing Atf4 expression prevented the OPA1-loss-induced tightening of MERC structures. OPA1 reduction was associated with decreased mitochondrial and sarcoplasmic reticulum, a specialized form of ER, calcium, which was reversed following ATF4 repression. These data suggest that mitochondrial stress, induced by OPA1 deficiency, regulates skeletal muscle MERC formation in an ATF4-dependent manner.
Keywords: activating transcription factor 4; endoplasmic reticulum; integrated stress response; interorganelle communication; mitochondria.
© 2024 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Figures



References
-
- Amodio G, Pagliara V, Moltedo O, & Remondelli K. (2021). Structural and Functional Significance of the Endoplasmic Reticulum Unfolded Protein Response Transducers and Chaperones at the Mitochondria–ER Contacts: A Cancer Perspective. Frontiers in Cell and Developmental Biology, 9. https://www.frontiersin.org/articles/10.3389/fcell.2021.641194 - DOI - PMC - PubMed
-
- Anastasia I, Ilacqua N, Raimondi A, Lemieux P, Ghandehari-Alavijeh R, Faure G, Mekhedov SL, Williams KJ, Caicci F, Valle G, Giacomello M, Quiroga AD, Lehner R, Miksis MJ, Toth K, de Aguiar Vallim TQ, Koonin EV, Scorrano L, & Pellegrini K. (2021). Mitochondria-rough-ER contacts in the liver regulate systemic lipid homeostasis. Cell Reports, 34(11), 108873. 10.1016/j.celrep.2021.108873 - DOI - PubMed
-
- Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, & Hall MN (2013). Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proceedings of the National Academy of Sciences of the United States of America, 110(31), 12526–12534. 10.1073/pnas.1302455110 - DOI - PMC - PubMed
-
- Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, Iglewski M, Chiong M, Simmen T, Zorzano A, Hill JA, Rothermel BA, Szabadkai G, & Lavandero K. (2011). Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. Journal of Cell Science, 124(Pt 13), 2143–2152. 10.1242/jcs.080762 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- T32 HL007344/HL/NHLBI NIH HHS/United States
- R01 HL108932/HL/NHLBI NIH HHS/United States
- R01 DK092065/DK/NIDDK NIH HHS/United States
- T32 GM133353/GM/NIGMS NIH HHS/United States
- R01 NS107265/NS/NINDS NIH HHS/United States
- ZIA HL006221/ImNIH/Intramural NIH HHS/United States
- RF1 AG055549/AG/NIA NIH HHS/United States
- R01 HL108379/HL/NHLBI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- RF1 AG059093/AG/NIA NIH HHS/United States
- NH/NIH HHS/United States
- T32 DK007563/DK/NIDDK NIH HHS/United States
- R25 HL106365/HL/NHLBI NIH HHS/United States
- RF1 AG062135/AG/NIA NIH HHS/United States
- R01 HL157956/HL/NHLBI NIH HHS/United States
- T32 5T32GM133353/GF/NIH HHS/United States
- R01 DK125405/DK/NIDDK NIH HHS/United States
- R01 AG072899/AG/NIA NIH HHS/United States
- AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

