Passaged Articular Chondrocytes From the Superficial Zone and Deep Zone Can Regain Zone-Specific Properties After Redifferentiation
- PMID: 38419462
- PMCID: PMC11610717
- DOI: 10.1177/03635465241230031
Passaged Articular Chondrocytes From the Superficial Zone and Deep Zone Can Regain Zone-Specific Properties After Redifferentiation
Abstract
Background: Bioengineered cartilage is a developing therapeutic to repair cartilage defects. The matrix must be rich in collagen type II and aggrecan and mechanically competent, withstanding compressive and shearing loads. Biomechanical properties in native articular cartilage depend on the zonal architecture consisting of 3 zones: superficial, middle, and deep. The superficial zone chondrocytes produce lubricating proteoglycan-4, whereas the deep zone chondrocytes produce collagen type X, which allows for integration into the subchondral bone. Zonal and chondrogenic expression is lost after cell number expansion. Current cell-based therapies have limited capacity to regenerate the zonal structure of native cartilage.
Hypothesis: Both passaged superficial and deep zone chondrocytes at high density can form bioengineered cartilage that is rich in collagen type II and aggrecan; however, only passaged superficial zone-derived chondrocytes will express superficial zone-specific proteoglycan-4, and only passaged deep zone-derived chondrocytes will express deep zone-specific collagen type X.
Study design: Controlled laboratory study.
Methods: Superficial and deep zone chondrocytes were isolated from bovine joints, and zonal subpopulations were separately expanded in 2-dimensional culture. At passage 2, superficial and deep zone chondrocytes were seeded, separately, in scaffold-free 3-dimensional culture within agarose wells and cultured in redifferentiation media.
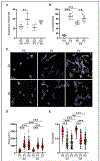
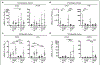
Results: Monolayer expansion resulted in loss of expression for proteoglycan-4 and collagen type X in passaged superficial and deep zone chondrocytes, respectively. By passage 2, superficial and deep zone chondrocytes had similar expression for dedifferentiated molecules collagen type I and tenascin C. Redifferentiation of both superficial and deep zone chondrocytes led to the expression of collagen type II and aggrecan in both passaged chondrocyte populations. However, only redifferentiated deep zone chondrocytes expressed collagen type X, and only redifferentiated superficial zone chondrocytes expressed and secreted proteoglycan-4. Additionally, redifferentiated deep zone chondrocytes produced a thicker and more robust tissue compared with superficial zone chondrocytes.
Conclusion: The recapitulation of the primary phenotype from passaged zonal chondrocytes introduces a novel method of functional bioengineering of cartilage that resembles the zone-specific biological properties of native cartilage.
Clinical relevance: The recapitulation of the primary phenotype in zonal chondrocytes could be a possible method to tailor bioengineered cartilage to have zone-specific expression.
Keywords: bioengineered cartilage; phenotypic memory; tissue engineering; zonal chondrocytes.
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: T.A.S. has a patent on rhPRG4, holds equity in Lubris BioPharma LLC, and is a paid consultant for Lubris LLC. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures



Similar articles
-
Zonal chondrocyte subpopulations reacquire zone-specific characteristics during in vitro redifferentiation.Am J Sports Med. 2009 Nov;37 Suppl 1:97S-104S. doi: 10.1177/0363546509350978. Epub 2009 Oct 21. Am J Sports Med. 2009. PMID: 19846691
-
Induced Redifferentiation of Human Chondrocytes from Articular Cartilage Lesion in Alginate Bead Culture After Monolayer Dedifferentiation: An Alternative Cell Source for Cell-Based Therapies?Tissue Eng Part A. 2018 Feb;24(3-4):275-286. doi: 10.1089/ten.TEA.2016.0505. Epub 2017 Jul 19. Tissue Eng Part A. 2018. PMID: 28610480
-
Dedifferentiation and redifferentiation of articular chondrocytes from surface and middle zones: changes in microRNAs-221/-222, -140, and -143/145 expression.Tissue Eng Part A. 2013 Apr;19(7-8):1015-22. doi: 10.1089/ten.TEA.2012.0055. Epub 2013 Feb 15. Tissue Eng Part A. 2013. PMID: 23190381
-
Perspective in Achieving Stratified Articular Cartilage Repair Using Zonal Chondrocytes.Tissue Eng Part B Rev. 2023 Jun;29(3):310-330. doi: 10.1089/ten.TEB.2022.0142. Epub 2023 Jan 24. Tissue Eng Part B Rev. 2023. PMID: 36416231 Review.
-
Bioengineering in the oral cavity: insights from articular cartilage tissue engineering.Int J Oral Maxillofac Implants. 2011;26 Suppl(Suppl):11-9; discussion 20-4. Int J Oral Maxillofac Implants. 2011. PMID: 21464997 Free PMC article. Review.
Cited by
-
Targeting the reorganization of F-actin for cell-based implantation cartilage repair therapies.Differentiation. 2025 May-Jun;143:100847. doi: 10.1016/j.diff.2025.100847. Epub 2025 Mar 1. Differentiation. 2025. PMID: 40068531 Review.
-
Targeting F-actin stress fibers to suppress the dedifferentiated phenotype in chondrocytes.Eur J Cell Biol. 2024 Jun;103(2):151424. doi: 10.1016/j.ejcb.2024.151424. Epub 2024 May 25. Eur J Cell Biol. 2024. PMID: 38823166 Free PMC article.
-
The Effects of Hypothermic Storage on Passaged Chondrocyte Viability and Redifferentiation Potential.bioRxiv [Preprint]. 2025 Jun 26:2025.06.20.660742. doi: 10.1101/2025.06.20.660742. bioRxiv. 2025. PMID: 40666901 Free PMC article. Preprint.
References
-
- Abubacker S, Dorosz SG, Ponjevic D, et al. Full-Length Recombinant Human Proteoglycan 4 Interacts with Hyaluronan to Provide Cartilage Boundary Lubrication. Ann Biomed Eng. 2016;44(4):1128–1137. - PubMed
-
- Antons J, Marascio MGM, Nohava J, et al. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J Mater Sci Mater Med. 2018;29(5):57. - PubMed
-
- Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol (Camb). 2012;4(4):410–421. - PubMed
-
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–224. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

