Evaluation of Ultrathin Strut Biodegradable Polymer-Coated Sirolimus-Eluting Stents in an All-Comers Patient Population: 1-Year Results of the S-FLEX Slovakia Registry
- PMID: 38419511
- PMCID: PMC10918283
- DOI: 10.14744/AnatolJCardiol.2023.3801
Evaluation of Ultrathin Strut Biodegradable Polymer-Coated Sirolimus-Eluting Stents in an All-Comers Patient Population: 1-Year Results of the S-FLEX Slovakia Registry
Abstract
Background: Supraflex (Sahajanand Medical Technologies Limited, Surat, India) is a new-generation, biodegradable polymer-coated sirolimus-eluting stent (SES) designed on an ultrathin (60 µm) cobalt-chromium platform with a flexible 'S-link.' The S-FLEX Slovakia registry aimed to assess the safety and effectiveness of Supraflex SES in an all-comers population, with a subgroup of diabetic patients.
Methods: This was a prospective, observational, multi-center, post-market registry conducted between February 2018 and May 2019. All consecutive patients with symptomatic coronary artery disease scheduled for percutaneous coronary intervention with Supraflex SES were enrolled. The primary endpoint was target lesion failure (TLF), defined as a composite of cardiac death, target vessel myocardial infarction (TV-MI), or clinically indicated target lesion revascularization (CI-TLR) by percutaneous or surgical methods at 1-year follow-up. Stent thrombosis was a safety endpoint.
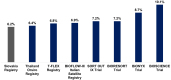
Results: A total of 413 patients was assessed (145 diabetics and 268 nondiabetics). At 1-year follow-up, the primary endpoint of TLF occurred in 5.1% patients, comprised of 3.9% cardiac deaths, 0.5% TV-MI, and 0.7% CI-TLR. Overall stent thrombosis occurred in 0.5% patients at 1-year follow-up. In the subgroup analysis, TLF occurred in 6.2% diabetics and 4.5% nondiabetics (P =.433) and comprised 4.8% and 3.4% cardiac deaths (P =.447), 0.7% and 0.4% TV-MI (P =.653), and 0.7%, and 0.7% CI-TLR (P =.952) in diabetics and non-diabetics, respectively. Overall stent thrombosis occurred in 0.7% diabetic and 0.4% non-diabetic patient (P =.659).
Conclusion: This registry demonstrates favourable clinical outcomes after the implantation of the ultrathin biodegradable polymer coated Supraflex SES in an all-comers population, with event rates that were similar in diabetic and nondiabetic patients.
Figures


Similar articles
-
Safety and performance of the ultrathin sirolimus-eluting coronary stent in an all-comer patient population: the S-FLEX UK-II registry.BMJ Open. 2024 Oct 22;14(10):e084028. doi: 10.1136/bmjopen-2024-084028. BMJ Open. 2024. PMID: 39438097 Free PMC article.
-
Clinical outcomes of ultrathin biodegradable polymer-coated sirolimus-eluting stents in an all-comer population: One-year results from the T-FLEX registry including high-risk subgroups.Anatol J Cardiol. 2021 Oct;25(10):706-715. doi: 10.5152/AnatolJCardiol.2021.78291. Anatol J Cardiol. 2021. PMID: 34622785 Free PMC article.
-
Clinical outcomes in 995 unselected real-world patients treated with an ultrathin biodegradable polymer-coated sirolimus-eluting stent: 12-month results from the FLEX Registry.BMJ Open. 2016 Feb 17;6(2):e010028. doi: 10.1136/bmjopen-2015-010028. BMJ Open. 2016. PMID: 26888727 Free PMC article.
-
Safety and efficacy of ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer drug-eluting stents: a meta-analysis of randomized trials.BMC Cardiovasc Disord. 2018 Aug 15;18(1):170. doi: 10.1186/s12872-018-0902-5. BMC Cardiovasc Disord. 2018. PMID: 30111289 Free PMC article. Review.
-
Long-term outcomes following ultrathin vs thin-strut drug-eluting stents for percutaneous coronary intervention: an updated systematic review and meta-analysis of randomized control trials.Am J Cardiovasc Dis. 2024 Oct 15;14(5):267-280. doi: 10.62347/UCLC9729. eCollection 2024. Am J Cardiovasc Dis. 2024. PMID: 39583995 Free PMC article. Review.
Cited by
-
Ultrathin Strut Biodegradable Polymer-Coated Sirolimus-Eluting Coronary Stents: Patient-Level Pooled Analysis From Two Indian Registries.Cureus. 2023 Jul 11;15(7):e41743. doi: 10.7759/cureus.41743. eCollection 2023 Jul. Cureus. 2023. PMID: 37575772 Free PMC article.
References
-
- Meier B. Forty years of percutaneous coronary intervention. Cardiovasc Med. 2018;21(11):278 281.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

