Prosopagnosia: face blindness and its association with neurological disorders
- PMID: 38419734
- PMCID: PMC10901275
- DOI: 10.1093/braincomms/fcae002
Prosopagnosia: face blindness and its association with neurological disorders
Abstract
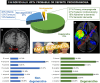
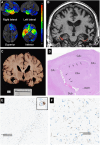
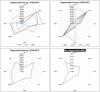
Loss of facial recognition or prosopagnosia has been well-recognized for over a century. It has been categorized as developmental or acquired depending on whether the onset is in early childhood or beyond, and acquired cases can have degenerative or non-degenerative aetiologies. Prosopagnosia has been linked to involvement of the fusiform gyri, mainly in the right hemisphere. The literature on prosopagnosia comprises case reports and small case series. We aim to assess demographic, clinical and imaging characteristics and neurological and neuropathological disorders associated with a diagnosis of prosopagnosia in a large cohort. Patients were categorized as developmental versus acquired; those with acquired prosopagnosia were further subdivided into degenerative versus non-degenerative, based on neurological aetiology. We assessed regional involvement on [18F] fluorodeoxyglucose-PET and MRI of the right and left frontal, temporal, parietal and occipital lobes. The Intake and Referral Center at the Mayo Clinic identified 487 patients with possible prosopagnosia, of which 336 met study criteria for probable or definite prosopagnosia. Ten patients, 80.0% male, had developmental prosopagnosia including one with Niemann-Pick type C and another with a forkhead box G1 gene mutation. Of the 326 with acquired prosopagnosia, 235 (72.1%) were categorized as degenerative, 91 (27.9%) as non-degenerative. The most common degenerative diagnoses were posterior cortical atrophy, primary prosopagnosia syndrome, Alzheimer's disease dementia and semantic dementia, with each diagnosis accounting for >10% of this group. The most common non-degenerative diagnoses were infarcts (ischaemic and haemorrhagic), epilepsy-related and primary brain tumours, each accounting for >10%. We identified a group of patients with non-degenerative transient prosopagnosia in which facial recognition loss improved or resolved over time. These patients had migraine-related prosopagnosia, posterior reversible encephalopathy syndrome, delirium, hypoxic encephalopathy and ischaemic infarcts. On [18F] fluorodeoxyglucose-PET, the temporal lobes proved to be the most frequently affected regions in 117 patients with degenerative prosopagnosia, while in 82 patients with non-degenerative prosopagnosia, MRI revealed the right temporal and right occipital lobes as most affected by a focal lesion. The most common pathological findings in those with degenerative prosopagnosia were frontotemporal lobar degeneration with hippocampal sclerosis and mixed Alzheimer's and Lewy body disease pathology. In this large case series of patients diagnosed with prosopagnosia, we observed that facial recognition loss occurs across a wide range of acquired degenerative and non-degenerative neurological disorders, most commonly in males with developmental prosopagnosia. The right temporal and occipital lobes, and connecting fusiform gyrus, are key areas. Multiple different pathologies cause degenerative prosopagnosia.
Keywords: MRI; PET; neurodegenerative; pathology; prosopagnosia.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
Kei.A.J. receives research support from the Mayo Clinic and from the National Institute of Health.
Figures


Similar articles
-
Right Fusiform Gyrus Infarct with Acute Prosopagnosia.Acta Neurol Taiwan. 2022 Dec 30;31(4):186-187. Acta Neurol Taiwan. 2022. PMID: 35470413
-
Structure and function in acquired prosopagnosia: lessons from a series of 10 patients with brain damage.J Neuropsychol. 2008 Mar;2(1):197-225. doi: 10.1348/174866407x214172. J Neuropsychol. 2008. PMID: 19334311
-
[Neurological disease and facial recognition].Brain Nerve. 2012 Jul;64(7):799-813. Brain Nerve. 2012. PMID: 22764352 Review. Japanese.
-
Understanding the functional neuroanatomy of acquired prosopagnosia.Neuroimage. 2007 Apr 1;35(2):836-52. doi: 10.1016/j.neuroimage.2006.09.051. Epub 2007 Feb 14. Neuroimage. 2007. PMID: 17303440
-
[Posterior cortical atrophy--a new dementia syndrome or a form of Alzheimer's disease?].Fortschr Neurol Psychiatr. 1996 Dec;64(12):492-508. doi: 10.1055/s-2007-996595. Fortschr Neurol Psychiatr. 1996. PMID: 9053390 Review. German.
References
-
- Bodamer J. [Prosop’s agnosia; the agnosia of cognition]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1947;118(1–2):6–53. Die Prosop-Agnosie; die Agnosie des Physiognomieerkennens. - PubMed
-
- Quaglino A, Borelli G. Emiplegia sinistra con amaurosi-guarigione-perdita totale delia percezione dei colori e della memoria della configurazione degli oggetti. Giornale d'Oftalmologia Italiano. 1867;10:106–117.
-
- Damasio AR, Damasio H, Van Hoesen GW. Prosopagnosia: Anatomic basis and behavioral mechanisms. Neurology. 1982;32(4):331–341. - PubMed
-
- Benton AL. The neuropsychology of facial recognition. Am Psychol. 1980;35(2):176–186. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
