p-Phenylenediamine-derived carbon nanodots for probing solvent interactions
- PMID: 38419862
- PMCID: PMC10898438
- DOI: 10.1039/d3na00799e
p-Phenylenediamine-derived carbon nanodots for probing solvent interactions
Abstract
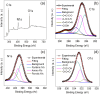
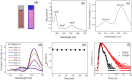
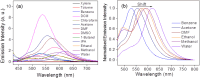
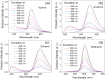
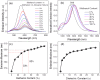
Carbon nanodots, the luminescent nanoparticles of carbon with size restriction below 10 nm, have attracted inordinate attention in materials science due to their widespread applications in optoelectronic and biological fields. Low toxicity and facile synthesis pathways render them favourites in the above-mentioned areas in the context of green chemistry. This work presents fine applications of p-phenylenediamine-derived carbon nanodots (PD-CNDs) achieved via a facile one-pot hydrothermal method. Adequate characterization using X-ray diffraction and spectroscopic and microscopic studies confirmed spherical particles with an average particle size of 2.8 nm, functionalised with amino, carboxyl, and hydroxyl groups. The carbon framework was functionalised with pyridinic and pyrrolic nitrogens. Upon 365 nm UV light illumination, an aqueous dispersion of PD-CNDs showed red-orange fluorescence. Detailed spectral analysis using UV-visible absorption and fluorescence spectroscopy identified edge states and surface groups as luminescent centres, with a significant contribution arising from the latter. The investigation conducted using a collection of solvents, categorized into polar and nonpolar, indicated the potential of the system for applications based on its solvatochromic nature. The feature enabled the determination of different polarity parameters of the solvents, as well as dielectric constants of solvents and solvent mixtures, with considerable accuracy. The system was potent for predicting the composition of a given pair of solvents. The service of the system is also extended for moisture sensing in organic solvents within an error percentage < 1. High quantum yield values (0.61) combined with solvent composition-dependent optical features ensure broader applications of the system to probe solvent interactions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Filik H. Avan A. A. Arabian J. Chem. 2020;13:6092–6105. doi: 10.1016/j.arabjc.2020.05.009. - DOI
-
- Serp P. and Machado B., Nanostructured Carbon Materials for Catalysis, The Royal Society of Chemistry, Cambridge, 2015
LinkOut - more resources
Full Text Sources

