Nanogold-coated stent facilitated non-invasive photothermal ablation of stent thrombosis and restoration of blood flow
- PMID: 38419863
- PMCID: PMC10898437
- DOI: 10.1039/d3na00751k
Nanogold-coated stent facilitated non-invasive photothermal ablation of stent thrombosis and restoration of blood flow
Abstract
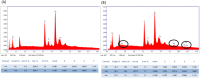
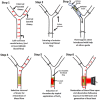
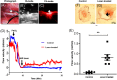
In-stent restenosis (ISR) and stent thrombosis (ST) are the most serious complications of coronary angioplasty and stenting. Although the evolution of drug-eluting stents (DES) has significantly restricted the incidence of ISR, they are associated with an enhanced risk of ST. In the present study, we explore the photothermal ablation of a thrombus using a nano-enhanced thermogenic stent (NETS) as a modality for revascularization following ST. The photothermal activity of NETS, fabricated by coating bare metal stents with gold nanorods generating a thin plasmonic film of gold, was found to be effective in rarefying clots formed within the stent lumen in various in vitro assays including those under conditions mimicking blood flow. NETS implanted in the rat common carotid artery generated heat following exposure to a NIR-laser that led to effective restoration of blood flow within the occluded vessel in a model of ferric chloride-induced thrombosis. Our results present a proof-of-concept for a novel photothermal ablation approach by employing coated stents in the non-invasive management of ST.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO, Cardiovascular diseases, https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1
-
- CDC, Heart Disease Deaths Vary by Sex, Race, and Ethnicity, https://www.cdc.gov/heartdisease/facts.htm#:∼:text=HeartDiseaseintheUnit...
LinkOut - more resources
Full Text Sources
Miscellaneous

