The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021-2022
- PMID: 38419967
- PMCID: PMC10900121
- DOI: 10.2903/j.efsa.2024.8583
The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2021-2022
Abstract
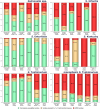
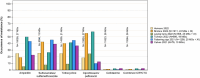
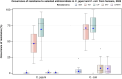
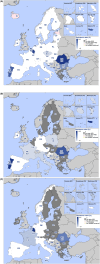
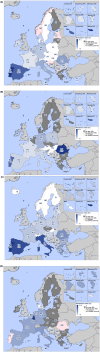
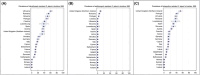
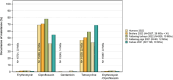
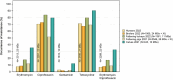
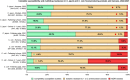
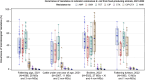
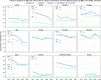
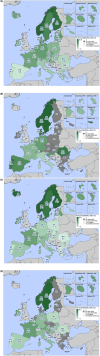
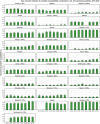
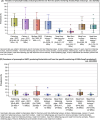
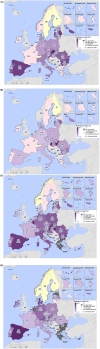
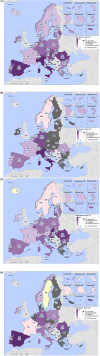
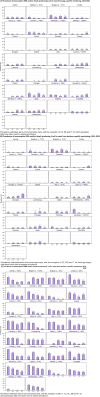
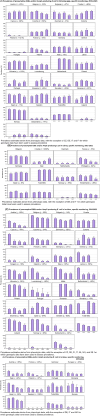
This report by the European Food Safety Authority and the European Centre for Disease prevention and Control, provides an overview of the main findings of the 2021-2022 harmonised Antimicrobial Resistance (AMR) monitoring in Salmonella spp., Campylobacter jejuni and C. coli from humans and food-producing animals (broilers, laying hens and fattening turkeys, fattening pigs and cattle under one year of age) and relevant meat thereof. For animals and meat thereof, AMR data on indicator commensal Escherichia coli, presumptive extended-spectrum beta-lactamases (ESBL)-/AmpC beta-lactamases (AmpC)-/carbapenemase (CP)-producing E. coli, and the occurrence of methicillin-resistant Staphylococcus aureus (MRSA) are also analysed. Generally, resistance levels differed greatly between reporting countries and antimicrobials. Resistance to commonly used antimicrobials was frequently found in Salmonella and Campylobacter isolates from humans and animals. In humans, increasing trends in resistance to one of two critically antimicrobials (CIA) for treatment was observed in poultry-associated Salmonella serovars and Campylobacter, in at least half of the reporting countries. Combined resistance to CIA was however observed at low levels except in some Salmonella serovars and in C. coli from humans and animals in some countries. While CP-producing Salmonella isolates were not detected in animals in 2021-2022, nor in 2021 for human cases, in 2022 five human cases of CP-producing Salmonella were reported (four harbouring bla OXA-48 or bla OXA-48-like genes). The reporting of a number of CP-producing E. coli isolates (harbouring bla OXA-48, bla OXA-181, bla NDM-5 and bla VIM-1 genes) in fattening pigs, cattle under 1 year of age, poultry and meat thereof by a limited number of MSs (5) in 2021 and 2022, requires a thorough follow-up. The temporal trend analyses in both key outcome indicators (rate of complete susceptibility and prevalence of ESBL-/AmpC-producers in E. coli) showed an encouraging progress in reducing AMR in food-producing animals in several EU MSs over the last 7 years.
Keywords: ESBL; MRSA; antimicrobial resistance; indicator bacteria; zoonotic bacteria.
© 2024 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Conflict of interest statement
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Figures
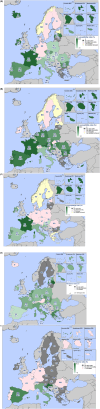

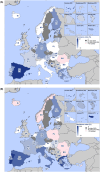
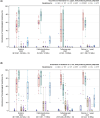




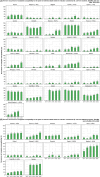
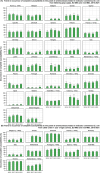

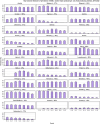
Broiler meat (DE, 2022): t011 (2), t034 (17), t571 (2), t2011 (1), t 2330 (1), t10485 (1), all CC398
Broiler meat (ES, 2022): CC398 spa‐type t6228 (1).
Turkey meat (DE, 2022): t008 CC8 (1), t127 CC5 (1), CC398 spa‐types t011 (16), t034 (85), t899 (26), t1255 (1), t1422 (3), t1430 (3), t1580 (1), t2011 (2), t5452 (2), t10204 (2).
Bovine meat (AT, 2021): CC45 spa‐type t095 (7), CC398 spa‐types t011 (4), t034 (2), CC121 spa‐type t898 (1), CC1 spa‐type t588 (1).
Bovine meat (DE, 2021): CC1 spa‐types t174 (1), t559 (1), CC9 spa‐type t1430 (1), CC97 spa‐types t359 (1), CC130 spa‐type t843 mecC positive (1), CC9/CC398 spa‐type t899 (1), CC398 spa‐types t011 (4), t034 (4), t1451 (1).
Pig meat (AT, 2021): CC9/CC398 spa‐type t899 (5), CC9 spa‐type t1430 (2), CC45 spa‐type t095 (11), CC398 spa‐types t011 (24), t034 (12), t1793 (1), t2576 (1), t9013 (1), t588 (1), t571 (1), no spa‐type reported (1).
Pig meat (FI, 2021): CC45 spa‐type t728 (1), CC398 spa‐types t034 (14), t899 (1), t2741 (9), t4677 (1).
Veal calves (BE, 2021): CC1 spa‐type t386 (1), CC398 spa‐types t011 (65), t034 (6), t1451 (1), t1456 (1), t2346 (1), t2370 (1), t3423 (1), t5210 (1), t6228 (1).
Dairy cows (BE, 2021): CC8 spa‐type t037 (3), CC398 spa‐types t011 (10), t034 (2).
Production cattle (BE, 2021): CC8 spa‐type t037 (2), CC398 spa‐type t011 (2).
Fattening pigs (BE, 2022): CC398 spa‐types t011 (63), t034 (26), t779 (1), t1255 (1), t1451 (1), t1580 (2), t2011 (2), t3423 (1), no spa‐type reported (1). Breeding pigs (BE, 2022): CC398 spa‐types t011 (46), t034 (16), t588 (1), t1451 (1), t1457 (1), t2011 (3), t3423 (1), t5104 (1), t6575 (1), t15528 (1).



Similar articles
-
The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021.EFSA J. 2023 Mar 6;21(3):e07867. doi: 10.2903/j.efsa.2023.7867. eCollection 2023 Mar. EFSA J. 2023. PMID: 36891283 Free PMC article.
-
The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019-2020.EFSA J. 2022 Mar 29;20(3):e07209. doi: 10.2903/j.efsa.2022.7209. eCollection 2022 Mar. EFSA J. 2022. PMID: 35382452 Free PMC article.
-
The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018.EFSA J. 2020 Mar 3;18(3):e06007. doi: 10.2903/j.efsa.2020.6007. eCollection 2020 Mar. EFSA J. 2020. PMID: 32874244 Free PMC article.
-
Harmonised monitoring of antimicrobial resistance in Salmonella and Campylobacter isolates from food animals in the European Union.Clin Microbiol Infect. 2008 Jun;14(6):522-33. doi: 10.1111/j.1469-0691.2008.02000.x. Epub 2008 Apr 5. Clin Microbiol Infect. 2008. PMID: 18397331 Review.
-
Review of antimicrobial resistance surveillance programmes in livestock and meat in EU with focus on humans.Clin Microbiol Infect. 2018 Jun;24(6):577-590. doi: 10.1016/j.cmi.2017.09.013. Epub 2017 Sep 29. Clin Microbiol Infect. 2018. PMID: 28970159 Review.
Cited by
-
Impact of Soil Fertilization with Pig Slurry on Antibiotic Residues and Resistance Genes: A Longitudinal Study.Antibiotics (Basel). 2024 May 24;13(6):486. doi: 10.3390/antibiotics13060486. Antibiotics (Basel). 2024. PMID: 38927154 Free PMC article.
-
Fecal Carriage and Risk Factors Associated with Extended-Spectrum β-Lactamase-/AmpC-/Carbapenemase-Producing Escherichia coli in Dogs from Italy.Animals (Basel). 2024 Nov 21;14(23):3359. doi: 10.3390/ani14233359. Animals (Basel). 2024. PMID: 39682325 Free PMC article.
-
Non-typhoidal Salmonella in food animals in Paraguay: predominant serovars and resistance phenotypes.Front Vet Sci. 2025 Mar 25;12:1521469. doi: 10.3389/fvets.2025.1521469. eCollection 2025. Front Vet Sci. 2025. PMID: 40201080 Free PMC article.
-
The role of veterinary drug use in driving antimicrobial resistance of Staphylococcus aureus isolates in smallholder swine farms in Central Vietnam.Open Vet J. 2025 Feb;15(2):847-862. doi: 10.5455/OVJ.2025.v15.i2.34. Epub 2025 Feb 28. Open Vet J. 2025. PMID: 40201839 Free PMC article.
-
Effect of feed supplementation with humic substances on phenotypic resistance profiles of Escherichia coli isolates from the ceca of broiler chickens.Poult Sci. 2025 Jul;104(7):105175. doi: 10.1016/j.psj.2025.105175. Epub 2025 Apr 16. Poult Sci. 2025. PMID: 40286573 Free PMC article.
References
-
- Abdullahi, I. N. , Fernández‐Fernández, R. , Juárez‐Fernández, G. , Martínez‐Álvarez, S. , Eguizábal, P. , Zarazaga, M. , Lozano, C. , & Torres, C. (2021). Wild animals are reservoirs and sentinels of Staphylococcus aureus and MRSA clones: A problem with “one health” concern. Antibiotics, 10(12), 1556. 10.3390/antibiotics10121556 - DOI - PMC - PubMed
-
- Adur, M. A. , Châtre, P. , Métayer, V. , Drapeau, A. , Pillonetto, M. , Penkal, M. L. , Lopes, J. K. , Beirão, B. C. B. , Madec, J.‐Y. , Freitas de Macedo, R. E. , & Haenni, M. (2022). Escherichia coli ST224 and IncF/blaCTX‐M‐55 plasmids drive resistance to extended‐spectrum cephalosporins in poultry flocks in Parana, Brazil. International Journal of Food Microbiology, 380(109885). 10.1016/j.ijfoodmicro.2022.109885 - DOI - PubMed
-
- Alba, P. , Feltrin, F. , Cordaro, G. , Porrero, M. C. , Kraushaar, B. , Argudín, M. A. , Nykäsenoja, S. , Monaco, M. , Stegger, M. , Aarestrup, F. M. , Butaye, P. , Franco, A. , & Battisti, A. (2015). Livestock‐associated methicillin resistant and methicillin susceptible Staphylococcus aureus sequence type (CC)1 in European farmed animals: High genetic relatedness of isolates from Italian cattle herds and humans. PLoS One, 10(8), e0137143. 10.1371/journal.pone.0137143 - DOI - PMC - PubMed
-
- Alba, P. , Leekitcharoenphon, P. , Carfora, V. , Amoruso, R. , Cordaro, G. , Di Matteo, P. , Ianzano, A. , Iurescia, M. , Diaconu, E. L. , Engage‐Eurl‐Ar Network Study Group , Pedersen, S. K. , Guerra, B. , Hendriksen, R. S. , Franco, A. , & Battisti, A. (2020). Molecular epidemiology of salmonella Infantis in Europe: Insights into the success of the bacterial host and its parasitic pESI‐like megaplasmid. Microbial. Genomics, 6(5), e000365. 10.1099/mgen.0.000365 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
