Programming temporal stiffness cues within extracellular matrix hydrogels for modelling cancer niches
- PMID: 38420142
- PMCID: PMC10900776
- DOI: 10.1016/j.mtbio.2024.101004
Programming temporal stiffness cues within extracellular matrix hydrogels for modelling cancer niches
Abstract
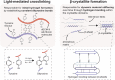
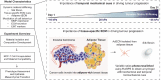
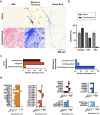
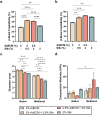
Extracellular matrix (ECM) stiffening is a common occurrence during the progression of many diseases, such as breast cancer. To accurately mimic the pathophysiological context of disease within 3D in vitro models, there is high demand for smart biomaterials which replicate the dynamic and temporal mechanical cues of diseased states. This study describes a preclinical disease model, using breast cancer as an example, which replicates the dynamic plasticity of the tumour microenvironment by incorporating temporal (3-week progression) biomechanical cues within a tissue-specific hydrogel microenvironment. The composite hydrogel formulation, integrating adipose-derived decellularised ECM (AdECM) and silk fibroin, was initially crosslinked using a visible light-mediated system, and then progressively stiffened through spontaneous secondary structure interactions inherent between the polymer chains (∼10-15 kPa increase, with a final stiffness of 25 kPa). When encapsulated and cultured in vitro, MCF-7 breast cancer cells initially formed numerous, large spheroids (>1000 μm2 in area), however, with progressive temporal stiffening, cells demonstrated growth arrest and underwent phenotypic changes resulting in intratumoral heterogeneity. Unlike widely-investigated static mechanical models, this stiffening hydrogel allowed for progressive phenotypic changes to be observed, and fostered the development of mature organoid-like spheroids, which mimicked both the organisation and acinar-structures of mature breast epithelium. The spheroids contained a central population of cells which expressed aggressive cellular programs, evidenced by increased fibronectin expression and reduction of E-cadherin. The phenotypic heterogeneity observed using this model is more reflective of physiological tumours, demonstrating the importance of establishing temporal cues within preclinical models in future work. Overall, the developed model demonstrated a novel strategy to uncouple ECM biomechanical properties from the cellular complexities of the disease microenvironment and offers the potential for wide applicability in other 3D in vitro disease models through addition of tissue-specific dECM materials.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Design of azobenzene-bearing hydrogel with photoswitchable mechanics driven by photo-induced phase transition for in vitro disease modeling.Acta Biomater. 2021 Sep 15;132:103-113. doi: 10.1016/j.actbio.2021.03.028. Epub 2021 Mar 17. Acta Biomater. 2021. PMID: 33744500
-
Electrostatic Assembly of Multiarm PEG-Based Hydrogels as Extracellular Matrix Mimics: Cell Response in the Presence and Absence of RGD Cell Adhesive Ligands.ACS Biomater Sci Eng. 2023 Mar 13;9(3):1362-1376. doi: 10.1021/acsbiomaterials.2c01252. Epub 2023 Feb 24. ACS Biomater Sci Eng. 2023. PMID: 36826383
-
3D extracellular matrix interactions modulate tumour cell growth, invasion and angiogenesis in engineered tumour microenvironments.Acta Biomater. 2016 May;36:73-85. doi: 10.1016/j.actbio.2016.03.017. Epub 2016 Mar 10. Acta Biomater. 2016. PMID: 26971667
-
Exploring the interaction between extracellular matrix components in a 3D organoid disease model to replicate the pathophysiology of breast cancer.J Exp Clin Cancer Res. 2023 Dec 16;42(1):343. doi: 10.1186/s13046-023-02926-4. J Exp Clin Cancer Res. 2023. PMID: 38102637 Free PMC article. Review.
-
Dynamic Hydrogels with Viscoelasticity and Tunable Stiffness for the Regulation of Cell Behavior and Fate.Materials (Basel). 2023 Jul 21;16(14):5161. doi: 10.3390/ma16145161. Materials (Basel). 2023. PMID: 37512435 Free PMC article. Review.
Cited by
-
Digital light processing of photoresponsive and programmable hydrogels.Sci Adv. 2025 Aug 8;11(32):eadw9262. doi: 10.1126/sciadv.adw9262. Epub 2025 Aug 8. Sci Adv. 2025. PMID: 40779636 Free PMC article.
-
Biomechanical Properties and Cellular Responses in Pulmonary Fibrosis.Bioengineering (Basel). 2024 Jul 24;11(8):747. doi: 10.3390/bioengineering11080747. Bioengineering (Basel). 2024. PMID: 39199705 Free PMC article. Review.
-
Development of tetraculture spheroids as a versatile 3D model for personalized breast cancer research.Sci Rep. 2025 Jul 28;15(1):27449. doi: 10.1038/s41598-025-12556-9. Sci Rep. 2025. PMID: 40721466 Free PMC article.
-
Growing Role of 3D In Vitro Cell Cultures in the Study of Cellular and Molecular Mechanisms: Short Focus on Breast Cancer, Endometriosis, Liver and Infectious Diseases.Cells. 2024 Jun 18;13(12):1054. doi: 10.3390/cells13121054. Cells. 2024. PMID: 38920683 Free PMC article. Review.
-
Myosin Light Chains in the Progression of Cancer.Cells. 2024 Dec 17;13(24):2081. doi: 10.3390/cells13242081. Cells. 2024. PMID: 39768172 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

