The impact of level of documentation on the accessibility and affordability of new drugs in Norway
- PMID: 38420198
- PMCID: PMC10899517
- DOI: 10.3389/fphar.2024.1338541
The impact of level of documentation on the accessibility and affordability of new drugs in Norway
Abstract
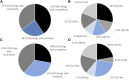
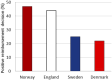
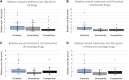
Introduction: Over the preceding decade, an increasing number of drugs have been approved by the European Medicines Agency (EMA) with limited knowledge of their relative efficacy. This is due to the utilization of non-randomized, single-arm studies, surrogate endpoints, and shorter follow-up time. The impact of this trend on the accessibility and affordability of newly approved drugs in Europe remains uncertain. The primary objective of this study is to provide insights into the issues of accessibility and affordability of new drugs in the Norwegian healthcare system. Method: The presented study entails an analysis of all reimbursement decisions for hospital drugs in Norway spanning 2021-2022. The included drugs were approved by the EMA between 2014 and 2022, with the majority (91%) receiving approval between 2018 and 2022. The drugs were categorized based on the level of documentation of relative efficacy. Approval rates and costs (confidential net-prices) were compared. Results: A total of 35% (70/199) of the reimbursement decisions were characterized by limited certainty regarding relative efficacy and as a consequence the Norwegian Health Technology Assessment (HTA) body did not present an incremental cost-effectiveness ratio (ICER) in the HTA report. Within this category, a lower percentage of drugs (47%) gained reimbursement approval compared to those with a higher certainty level, which were presented with an ICER (58%). On average, drugs with an established relative efficacy were accepted with a 4.4-fold higher cost (confidential net-prices). These trends persisted when specifically examining oncology drugs. Conclusion: Our study underscores that a substantial number of recently introduced drugs receive reimbursement regardless of the level of certainty concerning relative efficacy. However, the results suggest that payers prioritize documented over potential efficacy. Given that updated information on relative efficacy may emerge post-market access, a potential solution to address challenges related to accessibility and affordability in Europe could involve an increased adoption of market entry agreements. These agreements could allow for price adjustments after the presentation of new knowledge regarding relative efficacy, potentially resolving some of the current challenges.
Keywords: European Medicines Agency; Health Technology Assessment; drugs; managed-entry agreement; medicinal product; net-prices; oncology; reimbursement.
Copyright © 2024 Fagereng, Morvik, Reinvik Ulimoen, Ringerud, Dahlen Syversen and Sagdahl.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Beaver J. A., Howie L. J., Pelosof L., Kim T., Liu J., Goldberg K. B., et al. (2018). A 25-year experience of us food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 4 (6), 849–856. 10.1001/jamaoncol.2017.5618 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

