Real-time OCT feedback-controlled RPE photodisruption in ex vivo porcine eyes using 8 microsecond laser pulses
- PMID: 38420306
- PMCID: PMC10898567
- DOI: 10.1364/BOE.503941
Real-time OCT feedback-controlled RPE photodisruption in ex vivo porcine eyes using 8 microsecond laser pulses
Abstract
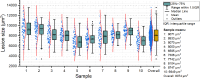
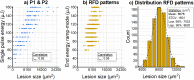
Selective retinal pigment epithelium (RPE) photodisruption requires reliable real-time feedback dosimetry (RFD) to prevent unwanted overexposure. In this study, optical coherence tomography (OCT) based RFD was investigated in ex vivo porcine eyes exposed to laser pulses of 8 µs duration (wavelength: 532 nm, exposure area: 90 × 90 µm2, radiant exposure: 247 to 1975 mJ/µm2). For RFD, fringe washouts in time-resolved OCT M-scans (central wavelength: 870 nm, scan rate: 85 kHz) were compared to an RPE cell viability assay. Statistical analysis revealed a moderate correlation between RPE lesion size and applied treatment energy, suggesting RFD adaptation to inter- and intraindividual RPE pigmentation and ocular transmission.
© 2023 Optica Publishing Group.
Conflict of interest statement
C.B. Meridian Medical (F,E,R), Heidelberg Engineering (F,R); The remaining authors declare no potential conflicts of interest.
Figures







Update of
- doi: 10.1364/opticaopen.24304489.
Similar articles
-
Investigation of the Influence of Pulse Duration and Application Mode on Microsecond Laser Microsurgery of the Retinal Pigment Epithelium.Life (Basel). 2023 Jun 2;13(6):1314. doi: 10.3390/life13061314. Life (Basel). 2023. PMID: 37374097 Free PMC article.
-
Selective Large-Area Retinal Pigment Epithelial Removal by Microsecond Laser in Preparation for Cell Therapy.Transl Vis Sci Technol. 2021 Aug 12;10(10):17. doi: 10.1167/tvst.10.10.17. Transl Vis Sci Technol. 2021. PMID: 34842907 Free PMC article.
-
Comparison of the tissue response of selective retina therapy with or without real-time feedback-controlled dosimetry.Graefes Arch Clin Exp Ophthalmol. 2018 Sep;256(9):1639-1651. doi: 10.1007/s00417-018-4067-9. Epub 2018 Jul 28. Graefes Arch Clin Exp Ophthalmol. 2018. PMID: 30056549
-
Targeting of the retinal pigment epithelium (RPE) by means of a rapidly scanned continuous wave (CW) laser beam.Lasers Surg Med. 2003;32(4):252-64. doi: 10.1002/lsm.10150. Lasers Surg Med. 2003. PMID: 12696092
-
Selective Retina Therapy.2019 Aug 14. In: Bille JF, editor. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics [Internet]. Cham (CH): Springer; 2019. Chapter 11. 2019 Aug 14. In: Bille JF, editor. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics [Internet]. Cham (CH): Springer; 2019. Chapter 11. PMID: 32091837 Free Books & Documents. Review.
References
-
- Szalai E., Nickerson J. M., Grossniklaus H. E., “RPE histopathology and morphometry,” in Retinal Pigment Epithelium in Health and Disease , Klettner A. K., Dithmar S., eds. (Springer Cham, 2020).10.1007/978-3-030-28384-1_1 - DOI
-
- Rizzolo L. J., “RPE polarity and barrier function,” in Retinal Pigment Epithelium in Health and Disease , Klettner A. K., Dithmar S., eds. (Springer Cham, 2020).10.1007/978-3-030-28384-1_2 - DOI
LinkOut - more resources
Full Text Sources
