Video-based sympathetic arousal assessment via peripheral blood flow estimation
- PMID: 38420320
- PMCID: PMC10898569
- DOI: 10.1364/BOE.507949
Video-based sympathetic arousal assessment via peripheral blood flow estimation
Abstract
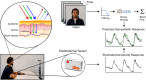
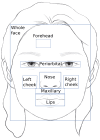
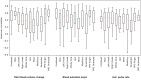
Electrodermal activity (EDA) is considered a standard marker of sympathetic activity. However, traditional EDA measurement requires electrodes in steady contact with the skin. Can sympathetic arousal be measured using only an optical sensor, such as an RGB camera? This paper presents a novel approach to infer sympathetic arousal by measuring the peripheral blood flow on the face or hand optically. We contribute a self-recorded dataset of 21 participants, comprising synchronized videos of participants' faces and palms and gold-standard EDA and photoplethysmography (PPG) signals. Our results show that we can measure peripheral sympathetic responses that closely correlate with the ground truth EDA. We obtain median correlations of 0.57 to 0.63 between our inferred signals and the ground truth EDA using only videos of the participants' palms or foreheads or PPG signals from the foreheads or fingers. We also show that sympathetic arousal is best inferred from the forehead, finger, or palm.
© 2023 Optica Publishing Group.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Electrodermal Activity Analysis at Different Body Locations.Sensors (Basel). 2025 Mar 12;25(6):1762. doi: 10.3390/s25061762. Sensors (Basel). 2025. PMID: 40292889 Free PMC article.
-
Highly sensitive index of sympathetic activity based on time-frequency spectral analysis of electrodermal activity.Am J Physiol Regul Integr Comp Physiol. 2016 Sep 1;311(3):R582-91. doi: 10.1152/ajpregu.00180.2016. Epub 2016 Jul 20. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27440716 Clinical Trial.
-
Wireless Measurement of Sympathetic Arousal During in vivo Occupational Therapy Sessions.Front Integr Neurosci. 2020 Oct 29;14:539875. doi: 10.3389/fnint.2020.539875. eCollection 2020. Front Integr Neurosci. 2020. PMID: 33192351 Free PMC article.
-
Current trends and opportunities in the methodology of electrodermal activity measurement.Physiol Meas. 2022 Mar 4;43(2). doi: 10.1088/1361-6579/ac5007. Physiol Meas. 2022. PMID: 35090148 Review.
-
[The measurement of electrodermal activity].Encephale. 2012 Apr;38(2):149-55. doi: 10.1016/j.encep.2011.05.004. Epub 2011 Oct 11. Encephale. 2012. PMID: 22516273 Review. French.
References
-
- Dawson M. E., Schell A. M., Filion D. L., The Electrodermal System (Cambridge University Press, 2016), pp. 217–243, Cambridge Handbooks in Psychology, 4th ed.
LinkOut - more resources
Full Text Sources
