Boosting open-label placebo effects in acute induced pain in healthy adults (BOLPAP-study): study protocol of a randomized controlled trial
- PMID: 38420356
- PMCID: PMC10900763
- DOI: 10.3389/fmed.2024.1238878
Boosting open-label placebo effects in acute induced pain in healthy adults (BOLPAP-study): study protocol of a randomized controlled trial
Abstract
Introduction: Pain is a highly prevalent symptom in the hospital setting, but treatment options remain limited. Harnessing the placebo effect in an ethical manner could provide a new possibility to reduce pain in clinical practice. So called open-label placebos (OLP) have been shown to elicit significant effects in reducing acute pain. But, before implementation, more knowledge concerning the properties of OLPs is needed. This study aims to assess the duration of analgesic effects from OLP and to determine the possibility of boosting such effects.
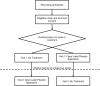
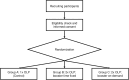
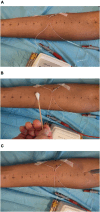
Methods and analysis: This is the protocol of an ongoing (first patient enrolled in March 2023) single-site randomized trial investigating OLPs in two parts (i.e., substudies). In both parts, pain will be induced in healthy adults using an intradermal electrical stimulation model. Participants in Part 1 will have two study visits: An interventional visit with one OLP injection accompanied by an evidence-based treatment rationale and a control visit with no treatment. For Part 2, participants will be randomized into three groups: (1) A fixed-time "Booster" group including one single repetition of the OLP injection at a fixed time point, (2) an on-demand "Booster" group including one single repetition of the OLP injection on-demand, and (3) a control group who will receive just one OLP injection. Differences in pain ratings over time (using the Numeric Rating Scale) will be analyzed with several two-sample t-tests. The time point for a fixed-time "Booster" in Part 2 will be derived from Part 1 with additional statistical tools such as a broken-stick mixed-effect model.
Discussion: This study aims to further characterize the analgesic effects of OLPs. In doing so, it will provide valuable information needed for later implementation of OLPs in clinical practice, where they could play a role in multimodal analgesic concepts.
Ethics and dissemination: The "Ethikkommission Nordwest- und Zentralschweiz" (BASEC 2023-00296) approved the study protocol. Results of the analysis will be submitted for publication in a peer-reviewed journal.
Clinical trial registration: This study is registered at ClinicalTrials.gov (NCT05819476) and is listed in the Swiss National Registry at kofam.ch (SNCTP000005470).
Keywords: acute pain; allodynia; booster; electrically-evoked pain; hyperalgesia; open-label placebo; placebo analgesia.
Copyright © 2024 de Leeuw, Laager, Gaab, Ruppen and Schneider.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Open-Label Placebo Treatment for Acute Postoperative Pain (OLP-POP Study): Study Protocol of a Randomized Controlled Trial.Front Med (Lausanne). 2021 Nov 5;8:687398. doi: 10.3389/fmed.2021.687398. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34805194 Free PMC article.
-
Can open label placebos improve pain and gluten tolerance via open label placebos in fibromyalgia patients? A study protocol for a randomised clinical trial in an outpatient centre.BMJ Open. 2023 Oct 21;13(10):e074957. doi: 10.1136/bmjopen-2023-074957. BMJ Open. 2023. PMID: 37865404 Free PMC article.
-
Open-label placebo treatment of women with premenstrual syndrome: study protocol of a randomised controlled trial.BMJ Open. 2020 Feb 17;10(2):e032868. doi: 10.1136/bmjopen-2019-032868. BMJ Open. 2020. PMID: 32071176 Free PMC article.
-
A systematic qualitative review of ethical issues in open label placebo in published research.Sci Rep. 2025 Apr 10;15(1):12268. doi: 10.1038/s41598-025-96425-5. Sci Rep. 2025. PMID: 40210672 Free PMC article.
-
Effects of open-label placebos in clinical trials: a systematic review and meta-analysis.Sci Rep. 2021 Feb 16;11(1):3855. doi: 10.1038/s41598-021-83148-6. Sci Rep. 2021. PMID: 33594150 Free PMC article.
References
-
- Michael M, Hossfeld B, Häske D, Bohn A, Bernhard M. Analgesie, sedierung und anästhesie in der notfallmedizin. Anästh Intensivmed. (2020) 61:051–63. 10.19224/ai2020.051 - DOI
Associated data
LinkOut - more resources
Full Text Sources
Medical

