N-glycoproteomic profiling reveals structural and functional alterations in yellow primary preserved egg white under saline-alkali treatment
- PMID: 38420501
- PMCID: PMC10900575
- DOI: 10.1016/j.fochx.2024.101244
N-glycoproteomic profiling reveals structural and functional alterations in yellow primary preserved egg white under saline-alkali treatment
Abstract
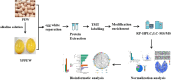
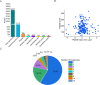
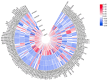
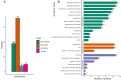
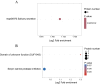
The posttranslational N-glycosylation of food proteins is important to their structure and function. However, the N-glycoproteomics of yellow preserved egg white were rarely reported. This study explored the changes of N-glycoproteome in yellow preserved eggs white after salt and alkali treatment. A total of 213 N-glycosites were identified on 102 glycoproteins, revealing prevalent glycosylation motifs and multiple N-glycosites within proteins. Salt and alkali treatment significantly altered the glycosylation patterns, impacting major proteins differently. GO analysis indicated the roles of differentially expressed glycoproteins in responding to stimuli and biological regulation. KEGG analysis emphasized the importance of salivary secretion pathway in enzyme secretion and peptide generation. Protein domain analysis highlighted the downregulation of Serpin. Protein-protein interaction networks revealed Apolipoprotein B as central players. This study provides essential structural information on the glycosylation modifications of egg white proteins, contributing to our understanding of the mechanisms behind the functional properties of preserved eggs.
Keywords: Gene Ontology analysis; N-Glycoproteome; Saline-alkali treatment; Yellow preserved primary egg white.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Gao B., Hu X., Li R., Zhao Y., Tu Y., Zhao Y. Screening of characteristic umami substances in preserved egg yolk based on the electronic tongue and UHPLC-MS/MS. LWT. 2021;152 doi: 10.1016/j.lwt.2021.112396. - DOI
LinkOut - more resources
Full Text Sources

