Downregulation of IRF8 in alveolar macrophages by G-CSF promotes metastatic tumor progression
- PMID: 38420590
- PMCID: PMC10901102
- DOI: 10.1016/j.isci.2024.109187
Downregulation of IRF8 in alveolar macrophages by G-CSF promotes metastatic tumor progression
Abstract
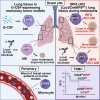
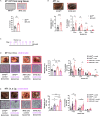
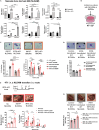
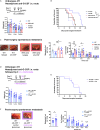
Tissue-resident macrophages (TRMs) are abundant immune cells within pre-metastatic sites, yet their functional contributions to metastasis remain incompletely understood. Here, we show that alveolar macrophages (AMs), the main TRMs of the lung, are susceptible to downregulation of the immune stimulatory transcription factor IRF8, impairing anti-metastatic activity in models of metastatic breast cancer. G-CSF is a key tumor-associated factor (TAF) that acts upon AMs to reduce IRF8 levels and facilitate metastasis. Translational relevance of IRF8 downregulation was observed among macrophage precursors in breast cancer and a CD68hiIRF8loG-CSFhi gene signature suggests poorer prognosis in triple-negative breast cancer (TNBC), a G-CSF-expressing subtype. Our data highlight the underappreciated, pro-metastatic roles of AMs in response to G-CSF and identify the contribution of IRF8-deficient AMs to metastatic burden. AMs are an attractive target of local neoadjuvant G-CSF blockade to recover anti-metastatic activity.
Keywords: Cancer; Immunology; Microenvironment.
© 2024 Roswell Park Comprehensive Cancer Center.
Conflict of interest statement
M.O. has received research support from Alphageneron, AIM Therapeutics, Eli Lilly, and Pfizer. The other authors declare no competing interests.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

