Survival and Quality of Life After Isolated Hepatic Perfusion With Melphalan as a Treatment for Uveal Melanoma Liver Metastases: Final Results From the Phase III Randomized Controlled Trial SCANDIUM
- PMID: 38420778
- PMCID: PMC12140551
- DOI: 10.1097/SLA.0000000000006255
Survival and Quality of Life After Isolated Hepatic Perfusion With Melphalan as a Treatment for Uveal Melanoma Liver Metastases: Final Results From the Phase III Randomized Controlled Trial SCANDIUM
Abstract
Objective: To investigate overall survival (OS) and health-related quality of life (HRQOL) of first-line isolated hepatic perfusion (IHP) compared to best alternative care for patients with uveal melanoma liver metastases.
Background: Approximately half of the patients with uveal melanoma develop metastatic disease, most commonly in the liver, and systemic treatment options are limited. IHP is a locoregional therapy with high response rates but with an unclear effect on OS.
Methods: In this phase III randomized controlled multicenter trial (the SCANDIUM trial), patients with previously untreated isolated uveal melanoma liver metastases were included between 2013 and 2021, with at least 24 months of follow-up. The planned accrual was 90 patients randomized 1:1 to receive a one-time treatment with IHP or best alternative care. Crossover to IHP was not allowed. The primary endpoint was the 24-month OS rate, with the hypothesis of a treatment effect leading to a 50% OS rate in the IHP group compared to 20% in the control group. HRQOL was measured by the EuroQol 5-domains 3-levels (EQ-5D-3L) questionnaire over 12 months.
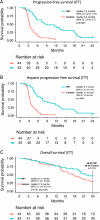
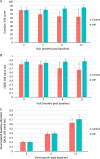
Results: The intention-to-treat population included 87 patients randomized to the IHP group [43 patients; 41 (89%) received IHP] or the control group (44 patients). The control group received chemotherapy (49%), immunotherapy (39%), or localized interventions (9%). In the intention-to-treat population, the median progression-free survival was 7.4 months in the IHP group compared with 3.3 months in the control group, with a hazard ratio of 0.21 (95% CI, 0.12-0.36). The 24-month OS rate was 46.5% in the IHP group versus 29.5% in the control group ( P =0.12). The median OS was 21.7 months versus 17.6 months, with a hazard ratio of 0.64 (95% CI, 0.37-1.10). EQ-5D-3L showed a sustained high health status for the IHP group over 12 months, compared to a deteriorating trend in the control group.
Conclusions: For patients with liver metastases from uveal melanoma, IHP offers high response rates translating to a benefit in progression-free survival including a trend of better HRQOL compared to the control group. However, the primary endpoint of OS at 24 months was not met.
Keywords: isolated hepatic perfusion; liver metastases; locoregional treatment; melphalan; uveal melanoma.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
R.O.B. has received institutional research grants from Bristol-Myers Squibb (BMS) and SkyLineDx, speaker honoraria from Roche and Pfizer, and has served on advisory boards for Amgen, BD/BARD, Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Novartis, Roche, and Sanofi Genzyme, and is a shareholder in SATMEG Ventures AB. L.N. has received institutional research grants from Merck and Syndax Pharmaceuticals, speaker honoraria from BMS, Johnson&Johnson, Leo Pharma, MSD, Novartis, and Pfizer, has served on advisory boards for BMS, MSD, Novartis, Pierre Fabre, Sanofi Genzyme, and Zealth, and is a shareholder in SATMEG Ventures AB. H.H. has received speaker honoraria from BMS, MSD, and Pierre Fabre and has served on advisory boards for MSD and Novartis. I.L.J. has served on advisory boards for MSD and BMS. J.N. is a shareholder in SATMEG Ventures AB. G.U. has during the past 2 years received honoraria for teaching activities by BMS, MSD, Pierre Fabre, and Novartis and consulting/advising by Incyte, BMS, Novartis, MSD, Alligator Bioscience, LIDDS, and SeqCure Immunology AB and is a stock owner in ESSITY and Oncopeptides. P.L. has served on advisory boards for Pierre Fabre. The remaining authors report no conflicts of interest.
Figures


Similar articles
-
Isolated Hepatic Perfusion With Melphalan for Patients With Isolated Uveal Melanoma Liver Metastases: A Multicenter, Randomized, Open-Label, Phase III Trial (the SCANDIUM Trial).J Clin Oncol. 2023 Jun 1;41(16):3042-3050. doi: 10.1200/JCO.22.01705. Epub 2023 Mar 20. J Clin Oncol. 2023. PMID: 36940407 Free PMC article. Clinical Trial.
-
Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver.Melanoma Res. 2004 Feb;14(1):67-72. doi: 10.1097/00008390-200402000-00011. Melanoma Res. 2004. PMID: 15091197 Clinical Trial.
-
Isolated hepatic perfusion for ocular melanoma metastasis: registry data suggests a survival benefit.Ann Surg Oncol. 2014 Feb;21(2):466-72. doi: 10.1245/s10434-013-3304-z. Epub 2013 Oct 19. Ann Surg Oncol. 2014. PMID: 24141377 Clinical Trial.
-
Optimizing the treatment of liver metastases from uveal melanomas with transarterial chemoembolization using melphalan and calibrated microspheres.Bull Cancer. 2020 Dec;107(12):1274-1283. doi: 10.1016/j.bulcan.2020.09.010. Epub 2020 Nov 9. Bull Cancer. 2020. PMID: 33183739 Review.
-
Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases.Cancers (Basel). 2021 Sep 21;13(18):4726. doi: 10.3390/cancers13184726. Cancers (Basel). 2021. PMID: 34572953 Free PMC article. Review.
Cited by
-
The Current State of Systemic Therapy of Metastatic Uveal Melanoma.Am J Clin Dermatol. 2024 Sep;25(5):691-700. doi: 10.1007/s40257-024-00872-1. Epub 2024 Jun 22. Am J Clin Dermatol. 2024. PMID: 38907174 Free PMC article. Review.
-
Optimizing TIL therapy for uveal melanoma: lessons learned and unlearned from cutaneous melanoma.Immunotherapy. 2025 Mar;17(4):283-291. doi: 10.1080/1750743X.2025.2478808. Epub 2025 Mar 18. Immunotherapy. 2025. PMID: 40098478 Free PMC article. Review.
-
Patient-derived xenografts and single-cell sequencing identifies three subtypes of tumor-reactive lymphocytes in uveal melanoma metastases.Elife. 2024 Sep 23;12:RP91705. doi: 10.7554/eLife.91705. Elife. 2024. PMID: 39312285 Free PMC article.
-
State-of-the-art in Metastatic Uveal Melanoma Treatment: A 2025 Update : How to treat Metastatic Uveal Melanoma in 2025.Curr Oncol Rep. 2025 Jul;27(7):803-821. doi: 10.1007/s11912-025-01684-0. Epub 2025 May 17. Curr Oncol Rep. 2025. PMID: 40380030 Free PMC article. Review.
-
Advances and Challenges in Immunotherapy for Metastatic Uveal Melanoma: Clinical Strategies and Emerging Targets.J Clin Med. 2025 Jul 19;14(14):5137. doi: 10.3390/jcm14145137. J Clin Med. 2025. PMID: 40725830 Free PMC article. Review.
References
-
- Diener-West M, Earle JD, Fine SL, et al. . The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001;119:969–982. - PubMed
-
- Piulats JM, Espinosa E, de la Cruz Merino L, et al. . Nivolumab plus ipilimumab for treatment-naive metastatic uveal melanoma: an open-label, multicenter, phase II trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J Clin Oncol. 2021;39:586–598. - PubMed
-
- Nathan P, Hassel JC, Rutkowski P, et al. . Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–1206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

