No-reflow after recanalization in ischemic stroke: From pathomechanisms to therapeutic strategies
- PMID: 38420850
- PMCID: PMC11318407
- DOI: 10.1177/0271678X241237159
No-reflow after recanalization in ischemic stroke: From pathomechanisms to therapeutic strategies
Abstract
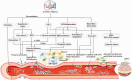
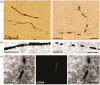
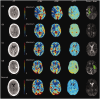
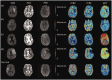
Endovascular reperfusion therapy is the primary strategy for acute ischemic stroke. No-reflow is a common phenomenon, which is defined as the failure of microcirculatory reperfusion despite clot removal by thrombolysis or mechanical embolization. It has been reported that up to 25% of ischemic strokes suffer from no-reflow, which strongly contributes to an increased risk of poor clinical outcomes. No-reflow is associated with functional and structural alterations of cerebrovascular microcirculation, and the injury to the microcirculation seriously hinders the neural functional recovery following macrovascular reperfusion. Accumulated evidence indicates that pathology of no-reflow is linked to adhesion, aggregation, and rolling of blood components along the endothelium, capillary stagnation with neutrophils, astrocytes end-feet, and endothelial cell edema, pericyte contraction, and vasoconstriction. Prevention or treatment strategies aim to alleviate or reverse these pathological changes, including targeted therapies such as cilostazol, adhesion molecule blocking antibodies, peroxisome proliferator-activated receptors (PPARs) activator, adenosine, pericyte regulators, as well as adjunctive therapies, such as extracorporeal counterpulsation, ischemic preconditioning, and alternative or complementary therapies. Herein, we provide an overview of pathomechanisms, predictive factors, diagnosis, and intervention strategies for no-reflow, and attempt to convey a new perspective on the clinical management of no-reflow post-ischemic stroke.
Keywords: No-reflow; ischemic stroke; pathogenesis; recanalization; therapy.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Microcirculation No-Reflow Phenomenon after Acute Ischemic Stroke.Eur Neurol. 2023;86(2):85-94. doi: 10.1159/000528250. Epub 2023 Jan 6. Eur Neurol. 2023. PMID: 36617418 Review.
-
No-reflow after stroke reperfusion therapy: An emerging phenomenon to be explored.CNS Neurosci Ther. 2024 Feb;30(2):e14631. doi: 10.1111/cns.14631. CNS Neurosci Ther. 2024. PMID: 38358074 Free PMC article. Review.
-
Quantitative imaging outperforms No-reflow in predicting functional outcomes in a translational stroke model.Neurotherapeutics. 2025 Mar;22(2):e00529. doi: 10.1016/j.neurot.2025.e00529. Epub 2025 Jan 31. Neurotherapeutics. 2025. PMID: 39893086 Free PMC article.
-
Pathological roles of mitochondrial dysfunction in endothelial cells during the cerebral no-reflow phenomenon: A review.Medicine (Baltimore). 2024 Dec 20;103(51):e40951. doi: 10.1097/MD.0000000000040951. Medicine (Baltimore). 2024. PMID: 39705421 Free PMC article. Review.
-
Prevention and treatment of no-reflow phenomenon by targeting the coronary microcirculation.Rev Cardiovasc Med. 2014;15(1):38-51. doi: 10.3909/ricm0699. Rev Cardiovasc Med. 2014. PMID: 24762465 Review.
Cited by
-
Adequate post-ischemic reperfusion of the mouse brain requires endothelial NFAT5.Acta Neuropathol Commun. 2024 Dec 22;12(1):200. doi: 10.1186/s40478-024-01918-5. Acta Neuropathol Commun. 2024. PMID: 39710754 Free PMC article.
-
Blood-Brain Barrier Leakage in the Penumbra Is Associated With Infarction on Follow-Up Imaging in Acute Ischemic Stroke.Stroke. 2025 Jul;56(7):1832-1842. doi: 10.1161/STROKEAHA.124.050171. Epub 2025 Apr 17. Stroke. 2025. PMID: 40242873 Free PMC article. Clinical Trial.
-
Brain border-derived CXCL2+ neutrophils drive NET formation and impair vascular reperfusion following ischemic stroke.CNS Neurosci Ther. 2024 Aug;30(8):e14916. doi: 10.1111/cns.14916. CNS Neurosci Ther. 2024. PMID: 39135337 Free PMC article.
-
A new taxonomy of neuroprotective agents for stroke appropriate for the reperfusion era.Front Neurol. 2025 Feb 5;15:1514924. doi: 10.3389/fneur.2024.1514924. eCollection 2024. Front Neurol. 2025. PMID: 40040642 Free PMC article.
-
Intra-Arterial Thrombolysis Following Endovascular Recanalization for Large Vessel Occlusion Stroke: A Systematic Review and Meta-Analysis.Neurology. 2025 Aug 12;105(3):e213842. doi: 10.1212/WNL.0000000000213842. Epub 2025 Jun 27. Neurology. 2025. PMID: 40577652 Free PMC article.
References
-
- Fischer EG, Ames RA, Hedley-Whyte ET, et al.. Reassessment of cerebral capillary changes in acute global ischemia and their relationship to the “no-reflow phenomenon”. Stroke 1977; 8: 36–39. - PubMed
-
- Eeckhout E, Kern MJ. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur Heart J 2001; 22: 729–739. - PubMed
-
- Law MM, Gelabert HA, Colburn MD, et al.. Continuous postoperative intra-arterial urokinase infusion in the treatment of no reflow following revascularization of the acutely ischemic limb. Ann Vasc Surg 1994; 8: 66–73. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

