Chemical and Cellular Formation of Reactive Oxygen Species from Secondary Organic Aerosols in Epithelial Lining Fluid
- PMID: 38420854
- PMCID: PMC10957138
Chemical and Cellular Formation of Reactive Oxygen Species from Secondary Organic Aerosols in Epithelial Lining Fluid
Abstract
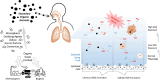
Introduction: Oxidative stress mediated by reactive oxygen species (ROS) is a key process for adverse aerosol health effects. Secondary organic aerosols (SOA) account for a major fraction of particulate matter with aerodynamic diameter ≤2.5 µm (PM2.5). PM2.5 inhalation and deposition into the respiratory tract causes the formation of ROS by chemical reactions and phagocytosis of macrophages in the epithelial lining fluid (ELF), but their relative contributions are not well quantified and their link to oxidative stress remains uncertain. The specific aims of this project were (1) elucidating the chemical mechanism and quantifying the formation kinetics of ROS in the ELF by SOA; (2) quantifying the relative importance of ROS formation by chemical reactions and macrophages in the ELF.
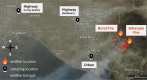
Methods: SOA particles were generated using reaction chambers from oxidation of various precursors including isoprene, terpenes, and aromatic compounds with or without nitrogen oxides (NOx). We collected size-segregated PM at two highway sites in Anaheim, CA, and Long Beach, CA, and at an urban site in Irvine, CA, during two wildfire events. The collected particles were extracted into water or surrogate ELF that contained lung antioxidants. ROS generation was quantified using electron paramagnetic resonance (EPR) spectroscopy with a spin-trapping technique. PM oxidative potential (OP) was also quantified using the dithiothreitol assay. In addition, kinetic modeling was applied for analysis and interpretation of experimental data. Finally, we quantified cellular superoxide release by RAW264.7 macrophage cells upon exposure to quinones and isoprene SOA using a chemiluminescence assay as calibrated with an EPR spin-probing technique. We also applied cellular imaging techniques to study the cellular mechanism of superoxide release and oxidative damage on cell membranes.
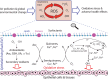
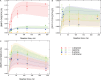
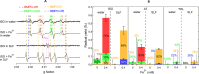
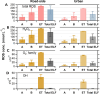
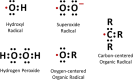
Results: Superoxide radicals (·O2-) were formed from aqueous reactions of biogenic SOA generated by hydroxy radical (·OH) photooxidation of isoprene, β-pinene, α-terpineol, and d-limonene. The temporal evolution of ·OH and ·O2- formation was elucidated by kinetic modeling with a cascade of aqueous reactions, including the decomposition of organic hydroperoxides (ROOH), ·OH oxidation of primary or secondary alcohols, and unimolecular decomposition of α-hydroxyperoxyl radicals. Relative yields of various types of ROS reflected the relative abundance of ROOH and alcohols contained in SOA, which generated under high NOx conditions, exhibited lower ROS yields. ROS formation by SOA was also affected by pH. Isoprene SOA had higher ·OH and organic radical yields at neutral than at acidic pH. At low pH ·O2- was the dominant species generated by all types of SOA. At neutral pH, α-terpineol SOA exhibited a substantial yield of carbon-centered organic radicals (R·), while no radical formation was observed by aromatic SOA.
Organic radicals in the ELF were formed by mixtures of Fe2+ and SOA generated from photooxidation of isoprene, α-terpineol, and toluene. The molar yields of organic radicals by SOA were 5-10 times higher in ELF than in water. Fe2+ enhanced organic radical yields by a factor of 20-80. Ascorbate mediated redox cycling of iron ions and sustained organic peroxide decomposition, as supported by kinetic modeling reproducing time- and concentration-dependence of organic radical formation, as well as by additional experiments observing the formation of Fe2+ and ascorbate radicals in mixtures of ascorbate and Fe3+. ·OH and superoxide were found to be efficiently scavenged by antioxidants.
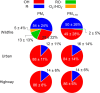
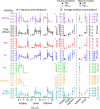
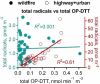
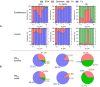
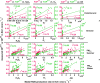
Wildfire PM mainly generated ·OH and R· with minor contributions from superoxide and oxygen-centered organic radicals (RO·). PM OP was high in wildfire PM, exhibiting very weak correlation with radical forms of ROS. These results were in stark contrast with PM collected at highway and urban sites, which generated much higher amounts of radicals dominated by ·OH radicals that correlated well with OP. By combining field measurements of size-segregated chemical composition, a human respiratory tract model, and kinetic modeling, we quantified production rates and concentrations of different types of ROS in different regions of the ELF by considering particle-size-dependent respiratory deposition. While hydrogen peroxide (H2O2) and ·O2- production were governed by Fe and Cu ions, ·OH radicals were mainly generated by organic compounds and Fenton-like reactions of metal ions. We obtained mixed results for correlations between PM OP and ROS formation, providing rationale and limitations of the use of oxidative potential as an indicator for PM toxicity in epidemiological and toxicological studies.
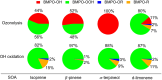
Quinones and isoprene SOA activated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in macrophages, releasing massive amounts of superoxide via respiratory burst and overwhelming the superoxide formation by aqueous chemical reactions in the ELF. The threshold dose for macrophage activation was much smaller for quinones compared with isoprene SOA. The released ROS caused lipid peroxidation to increase cell membrane fluidity, inducing oxidative damage and stress. Further increases of doses led to the activation of antioxidant response elements, reducing the net cellular superoxide production. At very high doses and long exposure times, chemical production became comparably important or dominant if the escalation of oxidative stress led to cell death.
Conclusions: The mechanistic understandings and quantitative information on ROS generation by SOA particles provided a basis for further elucidation of adverse aerosol health effects and oxidative stress by PM2.5. For a comprehensive assessment of PM toxicity and health effects via oxidative stress, it is important to consider both chemical reactions and cellular processes for the formation of ROS in the ELF. Chemical composition of PM strongly influences ROS formation; further investigations are required to study ROS formation from various PM sources. Such research will provide critical information to environmental agencies and policymakers for the development of air quality policy and regulation.
© 2023 Health Effects Institute. All rights reserved.
Figures







Similar articles
-
Understanding the Early Biological Effects of Isoprene-Derived Particulate Matter Enhanced by Anthropogenic Pollutants.Res Rep Health Eff Inst. 2019 Mar;2019(198):1-54. Res Rep Health Eff Inst. 2019. PMID: 31872748 Free PMC article.
-
Cellular and Acellular Assays for Measuring Oxidative Stress Induced by Ambient and Laboratory-Generated Aerosols.Res Rep Health Eff Inst. 2019 Mar;2019(197):1-57. Res Rep Health Eff Inst. 2019. PMID: 31872749 Free PMC article.
-
Superoxide Release by Macrophages through NADPH Oxidase Activation Dominating Chemistry by Isoprene Secondary Organic Aerosols and Quinones to Cause Oxidative Damage on Membranes.Environ Sci Technol. 2022 Dec 6;56(23):17029-17038. doi: 10.1021/acs.est.2c03987. Epub 2022 Nov 17. Environ Sci Technol. 2022. PMID: 36394988 Free PMC article.
-
A review of stereochemical implications in the generation of secondary organic aerosol from isoprene oxidation.Environ Sci Process Impacts. 2016 Nov 9;18(11):1369-1380. doi: 10.1039/c6em00354k. Environ Sci Process Impacts. 2016. PMID: 27762408 Review.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
Cited by
-
Professionalism vs. engagement: quality of SSc information on WeChat.Front Public Health. 2025 May 14;13:1527853. doi: 10.3389/fpubh.2025.1527853. eCollection 2025. Front Public Health. 2025. PMID: 40438054 Free PMC article.
References
-
- Abbas AK, Lichtman AH, Pillai S. 2010. Basic immunology. Functions and Disorders of the Immune system. Philadelphia, PA:Saunders Elsevier.
-
- Adams G, Boag J, Currant J, Michael B. 1965. Absolute Rate Constants for the Reaction of the Hydroxyl radical with Organic Compounds. New York:Academic Press.
-
- Anderson GH, Smith JG. 1968. Acid-catalyzed rearrangement of hydroperoxides. II. Phenylcycloalkyl hydroperoxides. Can J Chem 46:1561–1570.
-
- Arangio AM, Tong H, Socorro J, Pöschl U, Shiraiwa M. 2016. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles. Atmos Chem Phys 16:13105–13119.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
