Nucleotide metabolism, leukodystrophies, and CNS pathology
- PMID: 38421058
- PMCID: PMC11358362
- DOI: 10.1002/jimd.12721
Nucleotide metabolism, leukodystrophies, and CNS pathology
Abstract
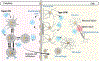
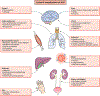
The balance between a protective and a destructive immune response can be precarious, as exemplified by inborn errors in nucleotide metabolism. This class of inherited disorders, which mimics infection, can result in systemic injury and severe neurologic outcomes. The most common of these disorders is Aicardi Goutières syndrome (AGS). AGS results in a phenotype similar to "TORCH" infections (Toxoplasma gondii, Other [Zika virus (ZIKV), human immunodeficiency virus (HIV)], Rubella virus, human Cytomegalovirus [HCMV], and Herpesviruses), but with sustained inflammation and ongoing potential for complications. AGS was first described in the early 1980s as familial clusters of "TORCH" infections, with severe neurology impairment, microcephaly, and basal ganglia calcifications (Aicardi & Goutières, Ann Neurol, 1984;15:49-54) and was associated with chronic cerebrospinal fluid (CSF) lymphocytosis and elevated type I interferon levels (Goutières et al., Ann Neurol, 1998;44:900-907). Since its first description, the clinical spectrum of AGS has dramatically expanded from the initial cohorts of children with severe impairment to including individuals with average intelligence and mild spastic paraparesis. This broad spectrum of potential clinical manifestations can result in a delayed diagnosis, which families cite as a major stressor. Additionally, a timely diagnosis is increasingly critical with emerging therapies targeting the interferon signaling pathway. Despite the many gains in understanding about AGS, there are still many gaps in our understanding of the cell-type drivers of pathology and characterization of modifying variables that influence clinical outcomes and achievement of timely diagnosis.
Keywords: Aicardi Goutieres syndrome; inborn error of metabolism; leukodystrophy; type I interferonopathy.
© 2024 SSIEM.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
LAA receives research support for research from Eli Lilly, Takeda, Illumina, Boehringer Ingelhiem, Biogen, Orchard Therapeutics. LAA serves on the scientific advisory boards of the MLD Foundation, CureMLD, and Don’t Forget Morgan Foundation. LAA is a consultant for Takeda, Biogen, and Orchard Therapeutics. AV receives grant and in-kind support for research from Eli Lilly, Gilead, Takeda, Illumina, Boehringer Ingelhiem, Biogen, Homology, Ionis, Passage Bio, Affinia, Sana, Sanofi, Myrtelle, Orchard Therapeutics. AV serves on the scientific advisory boards of the European Leukodystrophy Association and the United Leukodystrophy Foundation, as well as in an unpaid capacity for Takeda, Ionis, Biogen and Illumina. FG, CDG, KA, MS, LC, NM, AAD, and MB have no disclosures.
Figures

References
-
- Buchrieser J, Degrelle SA, Couderc T, et al. IFITM proteins inhibit placental syncytiotrophoblast formation and promote fetal demise. Science. 2019;365:176–180. - PubMed
-
- Aicardi J, Goutières F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49–54. - PubMed
-
- Goutières F, Aicardi J, Barth PG, Lebon P. Aicardi-Goutières syndrome: an update and results of interferon-alpha studies. Ann Neurol. 1998;44:900–907. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

