MKK3 K329 Mutation Attenuates Diabetes-Associated Cognitive Dysfunction by Blocking the MKK3-RAGE Interaction and Inhibiting Neuroinflammation
- PMID: 38421831
- PMCID: PMC11745445
- DOI: 10.14336/AD.2024.0222
MKK3 K329 Mutation Attenuates Diabetes-Associated Cognitive Dysfunction by Blocking the MKK3-RAGE Interaction and Inhibiting Neuroinflammation
Abstract
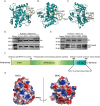
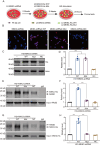
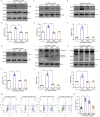
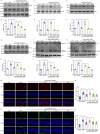
The receptor for advanced glycation end products (RAGE) contributes to diabetes-associated cognitive dysfunction (DACD) through the interaction of its C-terminal AAs 2-5 with mitogen-activated protein kinase kinase 3 (MKK3). However, the associated MKK3 binding site is unknown. Here, db/db mice were used as a model for type 2 diabetes. GST pull-down assays and AutoDock Vina simulations were conducted to identify the key RAGE binding site in MKK3. This binding site was mutated to investigate its effects on DACD and to elucidate the underlying mechanisms. The interaction of MKK3 and RAGE, the levels of inflammatory factors, and the activation of microglia and astrocytes were tested. Synaptic morphology and plasticity in hippocampal neurons were assessed via electrophysiological recordings and Golgi staining. Behavioral tests were used to assess cognitive function. In this study, MKK3 bound directly to RAGE via its lysine 329 (K329), leading to the activation of the nuclear factor-κB (NF-κB) signaling pathway, which in turn triggered neuroinflammation and synaptic dysfunction, and ultimately contributed to DACD. MKK3 mutation at K329 reversed synaptic dysfunction and cognitive deficits by downregulating the NF-κB signaling pathway and inhibiting neuroinflammation. These results confirm that neuroinflammation and synaptic dysfunction in the hippocampus rely on the direct binding of MKK3 and RAGE. We conclude that MKK3 K329 binding to C-terminal RAGE (ct-RAGE) is a key mechanism by which neuroinflammation and synaptic dysfunction are induced in the hippocampus. This study presents a novel mechanism for DACD and proposes a novel therapeutic avenue for neuroprotection in DACD.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Receptor for advanced glycation end products aggravates cognitive deficits in type 2 diabetes through binding of C-terminal AAs 2-5 to mitogen-activated protein kinase kinase 3 (MKK3) and facilitation of MEKK3-MKK3-p38 module assembly.Aging Cell. 2022 Feb;21(2):e13543. doi: 10.1111/acel.13543. Epub 2022 Jan 26. Aging Cell. 2022. PMID: 35080104 Free PMC article.
-
Binding of RAGE and RIPK1 induces cognitive deficits in chronic hyperglycemia-derived neuroinflammation.CNS Neurosci Ther. 2024 Mar;30(3):e14449. doi: 10.1111/cns.14449. Epub 2023 Sep 4. CNS Neurosci Ther. 2024. PMID: 37665158 Free PMC article.
-
Tanshinone IIA attenuates neuroinflammation via inhibiting RAGE/NF-κB signaling pathway in vivo and in vitro.J Neuroinflammation. 2020 Oct 14;17(1):302. doi: 10.1186/s12974-020-01981-4. J Neuroinflammation. 2020. PMID: 33054814 Free PMC article.
-
Aqueous extracts of se-enriched Auricularia auricular attenuates D-galactose-induced cognitive deficits, oxidative stress and neuroinflammation via suppressing RAGE/MAPK/NF-κB pathway.Neurosci Lett. 2019 Jun 21;704:106-111. doi: 10.1016/j.neulet.2019.04.002. Epub 2019 Apr 3. Neurosci Lett. 2019. PMID: 30953738
-
Astrocyte´s RAGE: More Than Just a Question of Mood.Cent Nerv Syst Agents Med Chem. 2018 Jan 26;18(1):39-48. doi: 10.2174/1871524916999160505105121. Cent Nerv Syst Agents Med Chem. 2018. PMID: 27149992 Review.
Cited by
-
The role of the Mediterranean diet in the treatment of cognitive dysfunction in patients with type 2 diabetes mellitus.Front Nutr. 2025 Aug 14;12:1654684. doi: 10.3389/fnut.2025.1654684. eCollection 2025. Front Nutr. 2025. PMID: 40896190 Free PMC article. No abstract available.
-
Jaranol alleviates cognitive impairment in db/db mice through the PI3K/AKT pathway.Metab Brain Dis. 2025 Jan 6;40(1):88. doi: 10.1007/s11011-024-01527-0. Metab Brain Dis. 2025. PMID: 39760807
References
-
- Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ (2022). Type 2 diabetes. Lancet, 400(10365): 1803-1820. - PubMed
-
- Cukierman T, Gerstein HC, Williamson JD (2005). Cognitive decline and dementia in diabetes-systematic overview of prospective observational studies. Diabetologia, 48: 2460-2469. - PubMed
-
- Dove A, Shang Y, Xu W, Grande G, Laukka EJ, Fratiglioni L, et al.. (2021). The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimers Dement, 17(11):1769-1778. - PubMed
-
- Biessels GJ, Nobili F, Teunissen CE, Simó R, Scheltens P (2020). Understanding multifactorial brain changes in type 2 diabetes: a biomarker perspective. Lancet Neurol, 19(8): 699-710. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
