Protocol for differentiation of functional macrophages from human induced pluripotent stem cells
- PMID: 38421862
- PMCID: PMC10910355
- DOI: 10.1016/j.xpro.2024.102925
Protocol for differentiation of functional macrophages from human induced pluripotent stem cells
Abstract
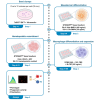
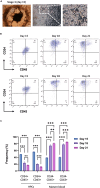
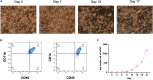
Human induced pluripotent stem cell (hiPSC)-derived macrophages provide a valuable tool for disease modeling and drug discovery. Here, we present a protocol to generate functional macrophages from hiPSCs using a feeder-free hematopoietic differentiation technique. We describe steps for preparing hiPSCs, mesodermal differentiation, hematopoietic commitment, and macrophage differentiation and expansion. We then detail assays to characterize their phenotype, polarization, and phagocytic functions. The functional macrophages generated here could be used to generate organoids for disease modeling and drug discovery studies. For complete details on the use and execution of this protocol, please refer to Jeong et al.1 and Heo et al.2.
Keywords: Cell Biology; Cell Differentiation; Cell culture; Cell isolation; Stem Cells.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The mesodermal induction with high dose of BMP4 and subsequent hematopoietic and macrophage differentiation are protected by published patents (KOR/10-2106710), and these intellectual property rights belong to KW-Bio Co., Ltd.
Figures




References
-
- Jeong S., An B., Kim J.H., Han H.W., Kim J.H., Heo H.R., Ha K.S., Han E.T., Park W.S., Hong S.H. BMP4 and perivascular cells promote hematopoietic differentiation of human pluripotent stem cells in a differentiation stage-specific manner. Exp. Mol. Med. 2020;52:56–65. doi: 10.1038/s12276-019-0357-5. - DOI - PMC - PubMed
-
- Heo H.R., Song H., Kim H.R., Lee J.E., Chung Y.G., Kim W.J., Yang S.R., Kim K.S., Chun T., Lee D.R., Hong S.H. Reprogramming mechanisms influence the maturation of hematopoietic progenitors from human pluripotent stem cells. Cell Death Dis. 2018;9:1090. doi: 10.1038/s41419-018-1124-6. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

