Neuron cilia restrain glial KCC-3 to a microdomain to regulate multisensory processing
- PMID: 38421867
- PMCID: PMC11296322
- DOI: 10.1016/j.celrep.2024.113844
Neuron cilia restrain glial KCC-3 to a microdomain to regulate multisensory processing
Abstract
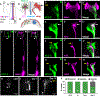
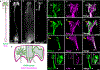
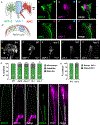
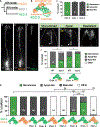
Glia interact with multiple neurons, but it is unclear whether their interactions with each neuron are different. Our interrogation at single-cell resolution reveals that a single glial cell exhibits specificity in its interactions with different contacting neurons. Briefly, C. elegans amphid sheath (AMsh) glia apical-like domains contact 12 neuron-endings. At these ad-neuronal membranes, AMsh glia localize the K/Cl transporter KCC-3 to a microdomain exclusively around the thermosensory AFD neuron to regulate its properties. Glial KCC-3 is transported to ad-neuronal regions, where distal cilia of non-AFD glia-associated chemosensory neurons constrain it to a microdomain at AFD-contacting glial membranes. Aberrant KCC-3 localization impacts both thermosensory (AFD) and chemosensory (non-AFD) neuron properties. Thus, neurons can interact non-synaptically through a shared glial cell by regulating microdomain localization of its cues. As AMsh and glia across species compartmentalize multiple cues like KCC-3, we posit that this may be a broadly conserved glial mechanism that modulates information processing across multimodal circuits.
Keywords: AFD; C. elegans; CP: Neuroscience; KCC-3; cell polarity; cilia; glia; glial microdomain; neuron-glia signaling; sensory processing.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Neuron cilia constrain glial regulators to microdomains around distal neurons.bioRxiv [Preprint]. 2023 Mar 18:2023.03.18.533255. doi: 10.1101/2023.03.18.533255. bioRxiv. 2023. PMID: 36993507 Free PMC article. Preprint.
-
A glial K(+) /Cl(-) cotransporter modifies temperature-evoked dynamics in Caenorhabditis elegans sensory neurons.Genes Brain Behav. 2016 Apr;15(4):429-40. doi: 10.1111/gbb.12260. Epub 2015 Nov 13. Genes Brain Behav. 2016. PMID: 26463820
-
A Glial K/Cl Transporter Controls Neuronal Receptive Ending Shape by Chloride Inhibition of an rGC.Cell. 2016 May 5;165(4):936-48. doi: 10.1016/j.cell.2016.03.026. Epub 2016 Apr 7. Cell. 2016. PMID: 27062922 Free PMC article.
-
Glia-Neuron Interactions in Caenorhabditis elegans.Annu Rev Neurosci. 2019 Jul 8;42:149-168. doi: 10.1146/annurev-neuro-070918-050314. Epub 2019 Mar 18. Annu Rev Neurosci. 2019. PMID: 30883261 Review.
-
Glial development and function in the nervous system of Caenorhabditis elegans.Cold Spring Harb Perspect Biol. 2015 Jan 8;7(4):a020578. doi: 10.1101/cshperspect.a020578. Cold Spring Harb Perspect Biol. 2015. PMID: 25573712 Free PMC article. Review.
Cited by
-
Glia Development and Function in the Nematode Caenorhabditis elegans.Cold Spring Harb Perspect Biol. 2024 Dec 2;16(12):a041346. doi: 10.1101/cshperspect.a041346. Cold Spring Harb Perspect Biol. 2024. PMID: 38565269 Free PMC article. Review.
-
The AFD-expressed SRTX-1 GPCR does not contribute to AFD thermosensory functions.MicroPubl Biol. 2024 Nov 13;2024:10.17912/micropub.biology.001382. doi: 10.17912/micropub.biology.001382. eCollection 2024. MicroPubl Biol. 2024. PMID: 39611104 Free PMC article.
-
Glia in Invertebrate Models: Insights from Caenorhabditis elegans.Adv Neurobiol. 2024;39:19-49. doi: 10.1007/978-3-031-64839-7_2. Adv Neurobiol. 2024. PMID: 39190070 Review.
-
Molecular profiling of invertebrate glia.Glia. 2025 Mar;73(3):632-656. doi: 10.1002/glia.24623. Epub 2024 Oct 16. Glia. 2025. PMID: 39415317 Free PMC article. Review.
-
Advances in Imaging Techniques for Mammalian/Human Ciliated Cell's Cilia: Insights into Structure, Function, and Dynamics.Biology (Basel). 2025 May 8;14(5):521. doi: 10.3390/biology14050521. Biology (Basel). 2025. PMID: 40427710 Free PMC article. Review.
References
-
- Eroglu Ç, Allen NJ, Susman MW, O’Rourke NA, Park CY, Özkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. (2009). Gabapentin Receptor α2δ−1 Is a Neuronal Thrombospondin Receptor Responsible for Excitatory CNS Synaptogenesis. Cell 139, 380–392. 10.1016/j.cell.2009.09.025. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

