Implications of MTHFD2 expression in renal cell carcinoma aggressiveness
- PMID: 38422037
- PMCID: PMC10903874
- DOI: 10.1371/journal.pone.0299353
Implications of MTHFD2 expression in renal cell carcinoma aggressiveness
Abstract
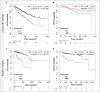
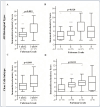
Renal cell carcinoma (RCC) is the most common type of cancer in kidney and is often diagnosed in advanced stages. Until now, there is no reliable biomarker to assess tumor prognosis during histopathological diagnosis. The Methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) overexpression has been suggested as prognostic indicator for RCC, however, its protein profile needs to be clarified. This study investigated the MTHFD2 expression in different RCC cohorts, associating it with tumor characteristics and prognostic factors. Gene expression comparisons between non-neoplastic (NN) and tumor samples, as well as patients' survival analysis, were assessed using KM-Plotter tool. MTHFD2 protein pattern was evaluated in 117 RCC by immunohistochemistry and associations with prognosis, clinical and pathological data were investigated. The tumors exhibited higher MTHFD2 transcript levels than NN, being even higher in the metastatic group. Opposite gene expression patterns were found among clear cell renal cell carcinoma (ccRCC) and pappilary renal cell carcinoma (pRCC) subtypes, showing higher and lower expressions compared to NN samples respectively. Overexpression was associated with shorter overall survival for ccRCC and pRCC subtypes, and shorter recurrence-free survival for pRCC. The immunolabeling profile varied according to tumor subtypes, with lower intensity and expression scores in ccRCC compared to pRCC and to chromophobe renal cell carcinoma (chRCC). MTHFD2 protein expression was associated with larger tumors and higher Fuhrman grades. Although prognostic value of protein immunostaining was not confirmed, patients with higher MTHFD2 tended to have lower survival rates in the pRCC group. The results highlight MTHFD2 different patterns according to RCC histological subtypes, revealing marked variations at both the genetic and protein levels. The mRNA indicated tumor prognosis, and greater expression in the tumor samples. Although MTHFD2 immunolabeling suggests tumor aggressiveness, it needs to be validated in other cohorts as potential prognostic factor.
Copyright: © 2024 Silva et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Uchendu IK, Tchawe YSN, Zhilenkova A V, Sangadzhieva ZD, Rusanov AS, Bagmet LN, et al.. Epidemiology Patterns of Renal Cell Carcinoma Worldwide: Examining Risk Factors and Contemporary Immunotherapy Approaches. 2023. doi: 10.20944/preprints202310.1757.v1 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

