Ongoing evolution of the Mycobacterium tuberculosis lactate dehydrogenase reveals the pleiotropic effects of bacterial adaption to host pressure
- PMID: 38422159
- PMCID: PMC10931510
- DOI: 10.1371/journal.ppat.1012050
Ongoing evolution of the Mycobacterium tuberculosis lactate dehydrogenase reveals the pleiotropic effects of bacterial adaption to host pressure
Abstract
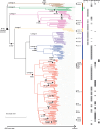
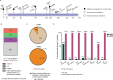
The bacterial determinants that facilitate Mycobacterium tuberculosis (Mtb) adaptation to the human host environment are poorly characterized. We have sought to decipher the pressures facing the bacterium in vivo by assessing Mtb genes that are under positive selection in clinical isolates. One of the strongest targets of selection in the Mtb genome is lldD2, which encodes a quinone-dependent L-lactate dehydrogenase (LldD2) that catalyzes the oxidation of lactate to pyruvate. Lactate accumulation is a salient feature of the intracellular environment during infection and lldD2 is essential for Mtb growth in macrophages. We determined the extent of lldD2 variation across a set of global clinical isolates and defined how prevalent mutations modulate Mtb fitness. We show the stepwise nature of lldD2 evolution that occurs as a result of ongoing lldD2 selection in the background of ancestral lineage-defining mutations and demonstrate that the genetic evolution of lldD2 additively augments Mtb growth in lactate. Using quinone-dependent antibiotic susceptibility as a functional reporter, we also find that the evolved lldD2 mutations functionally increase the quinone-dependent activity of LldD2. Using 13C-lactate metabolic flux tracing, we find that lldD2 is necessary for robust incorporation of lactate into central carbon metabolism. In the absence of lldD2, label preferentially accumulates in dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P) and is associated with a discernible growth defect, providing experimental evidence for accrued lactate toxicity via the deleterious buildup of sugar phosphates. The evolved lldD2 variants increase lactate incorporation to pyruvate while altering triose phosphate flux, suggesting both an anaplerotic and detoxification benefit to lldD2 evolution. We further show that the mycobacterial cell is transcriptionally sensitive to the changes associated with altered lldD2 activity which affect the expression of genes involved in cell wall lipid metabolism and the ESX- 1 virulence system. Together, these data illustrate a multifunctional role of LldD2 that provides context for the selective advantage of lldD2 mutations in adapting to host stress.
Copyright: © 2024 Stanley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Update of
-
Ongoing evolution of the Mycobacterium tuberculosis lactate dehydrogenase reveals the pleiotropic effects of bacterial adaption to host pressure.bioRxiv [Preprint]. 2023 Oct 9:2023.10.09.561592. doi: 10.1101/2023.10.09.561592. bioRxiv. 2023. Update in: PLoS Pathog. 2024 Feb 29;20(2):e1012050. doi: 10.1371/journal.ppat.1012050. PMID: 37873410 Free PMC article. Updated. Preprint.
References
-
- World Health Organization. (2022). Global tuberculosis report 2022.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

