Ebola virus VP35 interacts non-covalently with ubiquitin chains to promote viral replication
- PMID: 38422166
- PMCID: PMC10942258
- DOI: 10.1371/journal.pbio.3002544
Ebola virus VP35 interacts non-covalently with ubiquitin chains to promote viral replication
Abstract
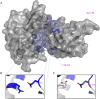
Ebolavirus (EBOV) belongs to a family of highly pathogenic viruses that cause severe hemorrhagic fever in humans. EBOV replication requires the activity of the viral polymerase complex, which includes the cofactor and Interferon antagonist VP35. We previously showed that the covalent ubiquitination of VP35 promotes virus replication by regulating interactions with the polymerase complex. In addition, VP35 can also interact non-covalently with ubiquitin (Ub); however, the function of this interaction is unknown. Here, we report that VP35 interacts with free (unanchored) K63-linked polyUb chains. Ectopic expression of Isopeptidase T (USP5), which is known to degrade unanchored polyUb chains, reduced VP35 association with Ub and correlated with diminished polymerase activity in a minigenome assay. Using computational methods, we modeled the VP35-Ub non-covalent interacting complex, identified the VP35-Ub interacting surface, and tested mutations to validate the interface. Docking simulations identified chemical compounds that can block VP35-Ub interactions leading to reduced viral polymerase activity. Treatment with the compounds reduced replication of infectious EBOV in cells and in vivo in a mouse model. In conclusion, we identified a novel role of unanchored polyUb in regulating Ebola virus polymerase function and discovered compounds that have promising anti-Ebola virus activity.
Copyright: © 2024 Rodríguez-Salazar et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Update of
-
Ebola Virus VP35 Interacts Non-Covalently with Ubiquitin Chains to Promote Viral Replication Creating New Therapeutic Opportunities.bioRxiv [Preprint]. 2023 Jul 15:2023.07.14.549057. doi: 10.1101/2023.07.14.549057. bioRxiv. 2023. Update in: PLoS Biol. 2024 Feb 29;22(2):e3002544. doi: 10.1371/journal.pbio.3002544. PMID: 37503276 Free PMC article. Updated. Preprint.
References
-
- Jacob ST, Crozier I, Fischer WA, Hewlett A, Kraft CS, de La Vega MA, et al. Ebola virus disease. Nat Rev Dis Primers [Internet]. 2020. Feb 20;6(1):13. Available from: https://www.nature.com/articles/s41572-020-0147-3 - PMC - PubMed
-
- Baseler L, Chertow DS, Johnson KM, Feldmann H, Morens DM. The Pathogenesis of Ebola Virus Disease. Annu Rev Pathol. 2017. Jan 24;12(1):387–418. Available from: https://www.annualreviews.org/doi/10.1146/annurev-pathol-052016-100506. - DOI - PubMed
-
- Malvy D, McElroy AK, de Clerck H, Günther S, van Griensven J. Ebola virus disease. Lancet [Internet]. 2019. Mar;393(10174):936–48. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673618331325. - PubMed
-
- Bharaj P, Atkins C, Luthra P, Giraldo MI, Dawes BE, Miorin L, et al. The Host E3-Ubiquitin Ligase TRIM6 Ubiquitinates the Ebola Virus VP35 Protein and Promotes Virus Replication. Lyles DS, editor. J Virol [Internet]. 2017. Sep 15;91(18):1–17. Available from: https://journals.asm.org/doi/10.1128/JVI.00833-17. - DOI - PMC - PubMed
-
- Leung DW, Ginder ND, Fulton DB, Nix J, Basler CF, Honzatko RB, et al. Structure of the Ebola VP35 interferon inhibitory domain. Proc Natl Acad Sci U S A. 2009. Jan 13;106(2):411–6. Available from: https://pnas.org/doi/full/10.1073/pnas.0807854106. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

