Induction of durable remission by dual immunotherapy in SHIV-infected ART-suppressed macaques
- PMID: 38422185
- PMCID: PMC11022498
- DOI: 10.1126/science.adf7966
Induction of durable remission by dual immunotherapy in SHIV-infected ART-suppressed macaques
Abstract
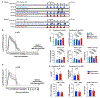
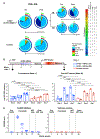
The eradication of the viral reservoir represents the major obstacle to the development of a clinical cure for established HIV-1 infection. Here, we demonstrate that the administration of N-803 (brand name Anktiva) and broadly neutralizing antibodies (bNAbs) results in sustained viral control after discontinuation of antiretroviral therapy (ART) in simian-human AD8 (SHIV-AD8)-infected, ART-suppressed rhesus macaques. N-803+bNAbs treatment induced immune activation and transient viremia but only limited reductions in the SHIV reservoir. Upon ART discontinuation, viral rebound occurred in all animals, which was followed by durable control in approximately 70% of all N-803+bNAb-treated macaques. Viral control was correlated with the reprogramming of CD8+ T cells by N-803+bNAb synergy. Thus, complete eradication of the replication-competent viral reservoir is likely not a prerequisite for the induction of sustained remission after discontinuation of ART.
Conflict of interest statement
Figures


References
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF, Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med 5, 512–517 (1999). doi:10.1038/8394 - DOI - PubMed
-
- Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson AS, Korber BT, Markowitz M, Ho DD, Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med 340, 1605–1613 (1999). doi:10.1056/NEJM199905273402101 - DOI - PubMed
-
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo Y-H, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF, Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188 (1997). doi:10.in38/387183an - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 OD011095/OD/NIH HHS/United States
- UM1 AI164565/AI/NIAID NIH HHS/United States
- R01 AI152703/AI/NIAID NIH HHS/United States
- P01 AI131365/AI/NIAID NIH HHS/United States
- R37 AI028433/AI/NIAID NIH HHS/United States
- R01 AI028433/AI/NIAID NIH HHS/United States
- P40 OD028116/OD/NIH HHS/United States
- R01 AI155163/AI/NIAID NIH HHS/United States
- P51 OD011106/OD/NIH HHS/United States
- R56 AI122942/AI/NIAID NIH HHS/United States
- R56 AI091514/AI/NIAID NIH HHS/United States
- R37 AI095098/AI/NIAID NIH HHS/United States
- UM1 AI144462/AI/NIAID NIH HHS/United States
- R01 AI161816/AI/NIAID NIH HHS/United States
- R01 AI127089/AI/NIAID NIH HHS/United States
- UM1 AI126617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

