Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression
- PMID: 38422473
- PMCID: PMC11107894
- DOI: 10.1200/JCO.23.01409
Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression
Abstract
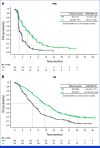
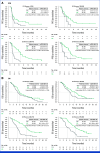
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.Sacituzumab govitecan (SG), a first-in-class anti-trophoblast cell surface antigen 2 (Trop-2) antibody-drug conjugate, demonstrated superior efficacy over single-agent chemotherapy (treatment of physician's choice [TPC]) in patients with metastatic triple-negative breast cancer (mTNBC) in the international, multicenter, phase III ASCENT study.Patients were randomly assigned 1:1 to receive SG or TPC until unacceptable toxicity/progression. Final efficacy secondary end point analyses and post hoc analyses of outcomes stratified by Trop-2 expression and human epidermal growth factor receptor 2 status are reported. Updated safety analyses are provided.In this final analysis, SG (n = 267) improved median progression-free survival (PFS; 4.8 v 1.7 months; hazard ratio (HR), 0.41 [95% CI, 0.33 to 0.52]) and median overall survival (OS; 11.8 v 6.9 months; HR, 0.51 [95% CI, 0.42 to 0.63]) over TPC (n = 262). SG improved PFS over TPC in each Trop-2 expression quartile (n = 168); a trend was observed for improved OS across quartiles. Overall, SG had a manageable safety profile, with ≤5% of treatment-related discontinuations because of adverse events and no treatment-related deaths. The safety profile was consistent across all subgroups.These data confirm the clinical benefit of SG over chemotherapy, reinforcing SG as an effective treatment option in patients with mTNBC in the second line or later.
Trial registration: ClinicalTrials.gov NCT02574455.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

