Comprehensive Inherited Risk Estimation for Risk-Based Breast Cancer Screening in Women
- PMID: 38422475
- PMCID: PMC11095905
- DOI: 10.1200/JCO.23.00295
Comprehensive Inherited Risk Estimation for Risk-Based Breast Cancer Screening in Women
Abstract
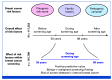
Purpose: Family history (FH) and pathogenic variants (PVs) are used for guiding risk surveillance in selected high-risk women but little is known about their impact for breast cancer screening on population level. In addition, polygenic risk scores (PRSs) have been shown to efficiently stratify breast cancer risk through combining information about common genetic factors into one measure.
Methods: In longitudinal real-life data, we evaluate PRS, FH, and PVs for stratified screening. Using FinnGen (N = 117,252), linked to the Mass Screening Registry for breast cancer (1992-2019; nationwide organized biennial screening for age 50-69 years), we assessed the screening performance of a breast cancer PRS and compared its performance with FH of breast cancer and PVs in moderate- (CHEK2)- to high-risk (PALB2) susceptibility genes.
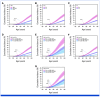
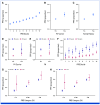
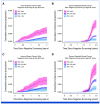
Results: Effect sizes for FH, PVs, and high PRS (>90th percentile) were comparable in screening-aged women, with similar implications for shifting age at screening onset. A high PRS identified women more likely to be diagnosed with breast cancer after a positive screening finding (positive predictive value [PPV], 39.5% [95% CI, 37.6 to 41.5]). Combinations of risk factors increased the PPVs up to 45% to 50%. A high PRS conferred an elevated risk of interval breast cancer (hazard ratio [HR], 2.78 [95% CI, 2.00 to 3.86] at age 50 years; HR, 2.48 [95% CI, 1.67 to 3.70] at age 60 years), and women with a low PRS (<10th percentile) had a low risk for both interval- and screen-detected breast cancers.
Conclusion: Using real-life screening data, this study demonstrates the effectiveness of a breast cancer PRS for risk stratification, alone and combined with FH and PVs. Further research is required to evaluate their impact in a prospective risk-stratified screening program, including cost-effectiveness.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Zielonke N, Gini A, Jansen EEL, et al. Evidence for reducing cancer-specific mortality due to screening for breast cancer in Europe: A systematic review. Eur J Cancer. 2020;127:191–206. - PubMed
-
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 19:77–102, 2021 - PubMed
-
- Sessa C, Balmana J, Bober SL, et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO clinical practice guideline. Ann Oncol. 2023;34:33–47. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

