Revised ISHAM-ABPA working group clinical practice guidelines for diagnosing, classifying and treating allergic bronchopulmonary aspergillosis/mycoses
- PMID: 38423624
- PMCID: PMC10991853
- DOI: 10.1183/13993003.00061-2024
Revised ISHAM-ABPA working group clinical practice guidelines for diagnosing, classifying and treating allergic bronchopulmonary aspergillosis/mycoses
Abstract
Background: The International Society for Human and Animal Mycology (ISHAM) working group proposed recommendations for managing allergic bronchopulmonary aspergillosis (ABPA) a decade ago. There is a need to update these recommendations due to advances in diagnostics and therapeutics.
Methods: An international expert group was convened to develop guidelines for managing ABPA (caused by Aspergillus spp.) and allergic bronchopulmonary mycosis (ABPM; caused by fungi other than Aspergillus spp.) in adults and children using a modified Delphi method (two online rounds and one in-person meeting). We defined consensus as ≥70% agreement or disagreement. The terms "recommend" and "suggest" are used when the consensus was ≥70% and <70%, respectively.
Results: We recommend screening for A. fumigatus sensitisation using fungus-specific IgE in all newly diagnosed asthmatic adults at tertiary care but only difficult-to-treat asthmatic children. We recommend diagnosing ABPA in those with predisposing conditions or compatible clinico-radiological presentation, with a mandatory demonstration of fungal sensitisation and serum total IgE ≥500 IU·mL-1 and two of the following: fungal-specific IgG, peripheral blood eosinophilia or suggestive imaging. ABPM is considered in those with an ABPA-like presentation but normal A. fumigatus-IgE. Additionally, diagnosing ABPM requires repeated growth of the causative fungus from sputum. We do not routinely recommend treating asymptomatic ABPA patients. We recommend oral prednisolone or itraconazole monotherapy for treating acute ABPA (newly diagnosed or exacerbation), with prednisolone and itraconazole combination only for treating recurrent ABPA exacerbations. We have devised an objective multidimensional criterion to assess treatment response.
Conclusion: We have framed consensus guidelines for diagnosing, classifying and treating ABPA/M for patient care and research.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: R. Agarwal has received grants from Cipla Pharmaceuticals, India for conducting research in ABPA. D.W. Denning and family hold founder shares in F2G Ltd, a University of Manchester spin-out antifungal discovery company, and share options in TFF Pharma. He acts or has recently acted as a consultant to Pulmatrix, Pulmocide, Biosergen, TFF Pharmaceuticals, Rostra Therapeutics, Mucpharma PTY and Lifemine Therapeutics. In the last 3 years, he has been paid for talks on behalf of Mundipharma, Bio-Rad, Basilea, Gilead, Avir and Pfizer. He has been involved in multiple guideline groups, primarily focused on diagnostics and aspergillosis. D. Armstrong-James holds share options in Pulmocide Ltd. K. Asano is partially supported by a research grant on allergic disease and immunology from the Japan Agency for Medical Research and Development under grant number 22ek0410097. S.H. Chotirmall has served on advisory boards for CSL Behring, Pneumagen Ltd and Boehringer Ingelheim, on data monitoring boards for Inovio Pharmaceuticals and Imam Abdulrahman Bin Faisal University, and has received personal fees from AstraZeneca and Chiesi Farmaceutici, all unrelated to this work. H.J.F. Salzer has received honoraria for lectures or consulting fees from Ismed, GlaxoSmithKline, AstraZeneca, Advanz Pharma, MSD and Chiesi, all unrelated to this work. J.D. Chalmers has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Grifols, Novartis, Insmed and Trudell, and received consultancy or speaker fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Insmed, Janssen, Novartis, Pfizer, Trudell and Zambon. C. Godet has received speaker fees and travel support from Pfizer and MSD, fees for board memberships from SOS Oxygène and Pulmatrix, and grant support from Ohre Pharma, Pfizer, MSD, SOS Oxygène, ISIS Medical, LVL Médical, Oxyvie, Vivisol, Elivie, CF Santé, Boehringer, Sandoz and AstraZeneca. M. Joest has received honoraria for lectures or grants from ALK-Abelló, AstraZeneca, Bencard, Berlin-Chemie, Boehringer Ingelheim, GlaxoSmithKline and HAL Allergy, all unrelated to this work. P. Nair reports grants and personal fees from AstraZeneca, Teva and Sanofi, personal fees from Equillium, Arrowhead Pharma and GlaxoSmithKline, and grants from Foresee and Cyclomedica, outside the submitted work. C.G. Baxter serves on strategic advisory board for Nob Hill Therapeutics Ltd. W.J. Calhoun is a member of the scientific advisory board of Pulmatrix, and that work is unrelated to this publication. O.A. Cornely reports grants from Cidara, F2G, Gilead, MSD, Mundipharma, Pfizer and Scynexis, consulting fees from AiCuris, Basilea, Cidara, Gilead, IQVIA, Matinas, Pfizer, PSI, Pulmocide and Scynexis, honoraria for lectures from Al-Jazeera, Gilead, Knight, MSD, Pfizer and Shionogi, and payment for expert testimony from Cidara. J.A. Douglass has received honoraria for educational presentations from AstraZeneca, GlaxoSmithKline, Novartis and CSL, has served on advisory boards for Sanofi-Aventis, Novartis, GlaxoSmithKline, AstraZeneca, Immunosis and CSL, and has undertaken contracted or investigator-initiated research on behalf of GlaxoSmithKline, Novartis, Immunosis, AstraZeneca, Sanofi-Aventis, Grifols, CSL, BioCryst and Equillium. R. Moss reports consulting fees from the Cystic Fibrosis Foundation, Astellas Pharma Inc., Nob Hill Therapeutics Inc., Pulmatrix, Mayne Pharma, Zambon Company SpA, Aridis Pharmaceuticals Inc., Regeneron Pharmaceuticals Inc. and 4D Molecular Therapeutics, royalties from UpToDate, Inc., and is a member of the board of directors for the Cystic Fibrosis Research Institute. D. Seidel received speaker fees from Pfizer, outside of the submitted work. R. Sprute has received speaker fees from Hikma and Pfizer, and travel support from Pfizer, all outside of the submitted work. The remaining authors have no potential conflicts of interest to disclose.
Figures
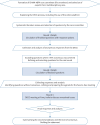
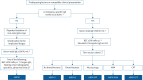
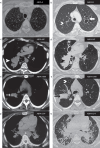
Comment in
-
2024 imaging criteria for allergic bronchopulmonary aspergillosis: which diagnostic cut-offs? Are chest radiograph and CT comparable?Eur Respir J. 2025 Apr 3;65(4):2500089. doi: 10.1183/13993003.00089-2025. Print 2025 Apr. Eur Respir J. 2025. PMID: 40180359 No abstract available.
-
Reply: Perspectives on high-attenuation mucus, CT and MRI in allergic bronchopulmonary aspergillosis.Eur Respir J. 2025 Apr 3;65(4):2500196. doi: 10.1183/13993003.00196-2025. Print 2025 Apr. Eur Respir J. 2025. PMID: 40180363 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
