Rational design of oligonucleotides for enhanced in vitro transcription of small RNA
- PMID: 38423625
- PMCID: PMC11098460
- DOI: 10.1261/rna.079923.123
Rational design of oligonucleotides for enhanced in vitro transcription of small RNA
Abstract
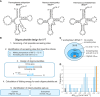
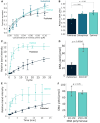
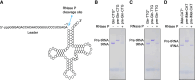
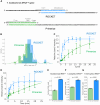
All kinds of RNA molecules can be produced by in vitro transcription using T7 RNA polymerase using DNA templates obtained by solid-phase chemical synthesis, primer extension, PCR, or DNA cloning. The oligonucleotide design, however, is a challenge to nonexperts as this relies on a set of rules that have been established empirically over time. Here, we describe a Python program to facilitate the rational design of oligonucleotides, calculated with kinetic parameters for enhanced in vitro transcription (ROCKET). The Python tool uses thermodynamic parameters, performs folding-energy calculations, and selects oligonucleotides suitable for the polymerase extension reaction. These oligonucleotides improve yields of template DNA. With the oligonucleotides selected by the program, the tRNA transcripts can be prepared by a one-pot reaction of the DNA polymerase extension reaction and the transcription reaction. Also, the ROCKET-selected oligonucleotides provide greater transcription yields than that from oligonucleotides selected by Primerize, a leading software for designing oligonucleotides for in vitro transcription, due to the enhancement of template DNA synthesis. Apart from over 50 tRNA genes tested, an in vitro transcribed self-cleaving ribozyme was found to have catalytic activity. In addition, the program can be applied to the synthesis of mRNA, demonstrating the wide applicability of the ROCKET software.
Keywords: ROCKET; in vitro transcription; oligonucleotide design; tRNA; thermodynamic parameters.
© 2024 Matsuda et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures






Similar articles
-
Single-pass transcription by T7 RNA polymerase.RNA. 2020 Dec;26(12):2062-2071. doi: 10.1261/rna.076778.120. Epub 2020 Sep 21. RNA. 2020. PMID: 32958559 Free PMC article.
-
Ribozyme processed tRNA transcripts with unfriendly internal promoter for T7 RNA polymerase: production and activity.FEBS Lett. 1998 Sep 25;436(1):99-103. doi: 10.1016/s0014-5793(98)01096-5. FEBS Lett. 1998. PMID: 9771901
-
Facilitated in vivo synthesis of ribonucleic acid and protein via T7 RNA polymerase.Anal Biochem. 2008 Apr 1;375(1):97-104. doi: 10.1016/j.ab.2007.11.037. Epub 2007 Dec 7. Anal Biochem. 2008. PMID: 18162164
-
Recent Advances in Biocatalytic and Chemoenzymatic Synthesis of Oligonucleotides.Chembiochem. 2025 May 5;26(9):e202400987. doi: 10.1002/cbic.202400987. Epub 2025 Feb 11. Chembiochem. 2025. PMID: 39854143 Review.
-
Circular oligonucleotides: new concepts in oligonucleotide design.Annu Rev Biophys Biomol Struct. 1996;25:1-28. doi: 10.1146/annurev.bb.25.060196.000245. Annu Rev Biophys Biomol Struct. 1996. PMID: 8800462 Review.
Cited by
-
A transfer RNA methyltransferase with an unusual domain composition catalyzes 2'-O-methylation at position 6 in tRNA.Nucleic Acids Res. 2025 Jul 8;53(13):gkaf579. doi: 10.1093/nar/gkaf579. Nucleic Acids Res. 2025. PMID: 40650981 Free PMC article.
-
tRNA pseudouridine synthase D (TruD) from Thermus thermophilus modifies U13 in tRNAAsp, tRNAGlu, and tRNAGln and U35 in tRNATyr.RNA. 2025 May 16;31(6):850-867. doi: 10.1261/rna.080405.125. RNA. 2025. PMID: 40138658
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
