Protective role of the HSP90 inhibitor, STA-9090, in lungs of SARS-CoV-2-infected Syrian golden hamsters
- PMID: 38423952
- PMCID: PMC10910676
- DOI: 10.1136/bmjresp-2023-001762
Protective role of the HSP90 inhibitor, STA-9090, in lungs of SARS-CoV-2-infected Syrian golden hamsters
Abstract
Introduction: The emergence of new SARS-CoV-2 variants, capable of escaping the humoral immunity acquired by the available vaccines, together with waning immunity and vaccine hesitancy, challenges the efficacy of the vaccination strategy in fighting COVID-19. Improved therapeutic strategies are urgently needed to better intervene particularly in severe cases of the disease. They should aim at controlling the hyperinflammatory state generated on infection, reducing lung tissue pathology and inhibiting viral replication. Previous research has pointed to a possible role for the chaperone HSP90 in SARS-CoV-2 replication and COVID-19 pathogenesis. Pharmacological intervention through HSP90 inhibitors was shown to be beneficial in the treatment of inflammatory diseases, infections and reducing replication of diverse viruses.
Methods: In this study, we investigated the effects of the potent HSP90 inhibitor Ganetespib (STA-9090) in vitro on alveolar epithelial cells and alveolar macrophages to characterise its effects on cell activation and viral replication. Additionally, the Syrian hamster animal model was used to evaluate its efficacy in controlling systemic inflammation and viral burden after infection.
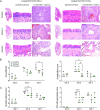
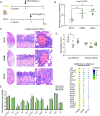
Results: In vitro, STA-9090 reduced viral replication on alveolar epithelial cells in a dose-dependent manner and lowered significantly the expression of proinflammatory genes, in both alveolar epithelial cells and alveolar macrophages. In vivo, although no reduction in viral load was observed, administration of STA-9090 led to an overall improvement of the clinical condition of infected animals, with reduced oedema formation and lung tissue pathology.
Conclusion: Altogether, we show that HSP90 inhibition could serve as a potential treatment option for moderate and severe cases of COVID-19.
Keywords: COVID-19; inflammation; pneumonia; respiratory infection.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- WHO . Coronavirus disease (COVID-19) pandemic, . 2021.. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
