Deletion of the sugar importer gene OsSWEET1b accelerates sugar starvation-promoted leaf senescence in rice
- PMID: 38423969
- PMCID: PMC11213257
- DOI: 10.1093/plphys/kiae098
Deletion of the sugar importer gene OsSWEET1b accelerates sugar starvation-promoted leaf senescence in rice
Abstract
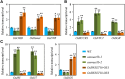
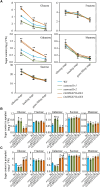
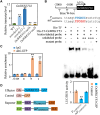
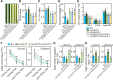
Leaf senescence is a combined response of plant cells stimulated by internal and external signals. Sugars acting as signaling molecules or energy metabolites can influence the progression of leaf senescence. Both sugar starvation and accumulation can promote leaf senescence with diverse mechanisms that are reported in different species. Sugars Will Eventually be Exported Transporters (SWEETs) are proposed to play essential roles in sugar transport, but whether they have roles in senescence and the corresponding mechanism are unclear. Here, we functionally characterized a sugar transporter, OsSWEET1b, which transports sugar and promotes senescence in rice (Oryza sativa L.). OsSWEET1b could import glucose and galactose when heterologously expressed in Xenopus oocytes and translocate glucose and galactose from the extracellular apoplast into the intracellular cytosol in rice. Loss of function of OsSWEET1b decreased glucose and galactose accumulation in leaves. ossweet1b mutants showed accelerated leaf senescence under natural and dark-induced conditions. Exogenous application of glucose and galactose complemented the defect of OsSWEET1b deletion-promoted senescence. Moreover, the senescence-activated transcription factor OsWRKY53, acting as a transcriptional repressor, genetically functions upstream of OsSWEET1b to suppress its expression. OsWRKY53-overexpressing plants had attenuated sugar accumulation, exhibiting a similar phenotype as the ossweet1b mutants. Our findings demonstrate that OsWRKY53 downregulates OsSWEET1b to impair its influx transport activity, leading to compromised sugar accumulation in the cytosol of rice leaves where sugar starvation promotes leaf senescence.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

