Mesoporous nanoperforators as membranolytic agents via nano- and molecular-scale multi-patterning
- PMID: 38424084
- PMCID: PMC10904871
- DOI: 10.1038/s41467-024-46189-9
Mesoporous nanoperforators as membranolytic agents via nano- and molecular-scale multi-patterning
Abstract
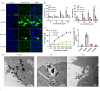
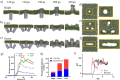
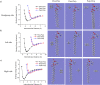
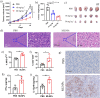
Plasma membrane lysis is an effective anticancer strategy, which mostly relying on soluble molecular membranolytic agents. However, nanomaterial-based membranolytic agents has been largely unexplored. Herein, we introduce a mesoporous membranolytic nanoperforators (MLNPs) via a nano- and molecular-scale multi-patterning strategy, featuring a spiky surface topography (nanoscale patterning) and molecular-level periodicity in the spikes with a benzene-bridged organosilica composition (molecular-scale patterning), which cooperatively endow an intrinsic membranolytic activity. Computational modelling reveals a nanospike-mediated multivalent perforation behaviour, i.e., multiple spikes induce nonlinearly enlarged membrane pores compared to a single spike, and that benzene groups aligned parallelly to a phospholipid molecule show considerably higher binding energy than other alignments, underpinning the importance of molecular ordering in phospholipid extraction for membranolysis. Finally, the antitumour activity of MLNPs is demonstrated in female Balb/c mouse models. This work demonstrates assembly of organosilica based bioactive nanostructures, enabling new understandings on nano-/molecular patterns co-governed nano-bio interaction.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Park NH, et al. Addressing drug resistance in cancer with macromolecular chemotherapeutic agents. J. Am. Chem. Soc. 2018;140:4244–4252. - PubMed
-
- Liu MD, et al. A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat. Nanotechnol. 2022;17:541–551. - PubMed
-
- Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 2006;6:940–952. - PubMed
-
- Zhou J, et al. Delivery strategies for melittin-based cancer therapy. ACS Appl. Mater. Interfaces. 2021;13:17158–17173. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

