Dual T-cell constant β chain (TRBC)1 and TRBC2 staining for the identification of T-cell neoplasms by flow cytometry
- PMID: 38424120
- PMCID: PMC10904869
- DOI: 10.1038/s41408-024-01002-0
Dual T-cell constant β chain (TRBC)1 and TRBC2 staining for the identification of T-cell neoplasms by flow cytometry
Abstract
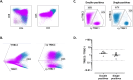
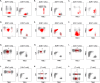
The diagnosis of leukemic T-cell malignancies is often challenging, due to overlapping features with reactive T-cells and limitations of currently available T-cell clonality assays. Recently developed therapeutic antibodies specific for the mutually exclusive T-cell receptor constant β chain (TRBC)1 and TRBC2 isoforms provide a unique opportunity to assess for TRBC-restriction as a surrogate of clonality in the flow cytometric analysis of T-cell neoplasms. To demonstrate the diagnostic utility of this approach, we studied 164 clinical specimens with (60) or without (104) T-cell neoplasia, in addition to 39 blood samples from healthy donors. Dual TRBC1 and TRBC2 expression was studied within a comprehensive T-cell panel, in a fashion similar to the routine evaluation of kappa and lambda immunoglobulin light chains for the detection of clonal B-cells. Polytypic TRBC expression was demonstrated on total, CD4+ and CD8+ T-cells from all healthy donors; and by intracellular staining on benign T-cell precursors. All neoplastic T-cells were TRBC-restricted, except for 8 cases (13%) lacking TRBC expression. T-cell clones of uncertain significance were identified in 17 samples without T-cell malignancy (13%) and accounted for smaller subsets than neoplastic clones (median: 4.7 vs. 69% of lymphocytes, p < 0.0001). Single staining for TRBC1 produced spurious TRBC1-dim subsets in 24 clinical specimens (15%), all of which resolved with dual TRBC1/2 staining. Assessment of TRBC restriction by flow cytometry provides a rapid diagnostic method to detect clonal T-cells, and to accurately determine the targetable TRBC isoform expressed by T-cell malignancies.
© 2024. The Author(s).
Conflict of interest statement
MP and PMM are inventors on a patent describing the use of TRBC1/2 for diagnosis and treatment of T-cell malignancies. MP and MF are inventors on a patent describing TRBC2 antibodies. Autolus Therapeutics owns patents claiming the use of TRBC1/2 for diagnosis. MP, MF, and ZA own stock in and are employees of Autolus Therapeutics. FTI is a former Autolus employee. The remaining authors declare no competing interests.
Figures




References
-
- Tembhare PR, Chatterjee G, Chaturvedi A, Dasgupta N, Khanka T, Verma S, et al. Critical role of flow cytometric immunophenotyping in the diagnosis, subtyping, and staging of T-cell/NK-cell non-Hodgkin’s lymphoma in real-world practice: a study of 232 cases from a tertiary cancer center in India. Front Oncol. 2022;12:779230. doi: 10.3389/fonc.2022.779230. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

