Long-term outcomes after upfront second-generation tyrosine kinase inhibitors for chronic myeloid leukemia: managing intolerance and resistance
- PMID: 38424138
- PMCID: PMC10997507
- DOI: 10.1038/s41375-024-02187-w
Long-term outcomes after upfront second-generation tyrosine kinase inhibitors for chronic myeloid leukemia: managing intolerance and resistance
Abstract
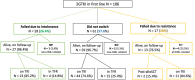
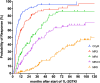
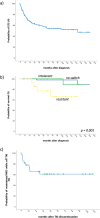
Second-generation tyrosine kinase inhibitors (2GTKI) are more effective in inducing rapid molecular responses than imatinib when used first-line in patients with chronic myeloid leukemia in chronic phase (CML-CP). However, failure of first line-2GTKI (1L-2GTKI) still occurs and there is no consensus regarding subsequent management. We retrospectively analyzed the outcome of 106 CML-CP patients treated with 1L-2GTKI and with a median follow-up of 91 months. 45 patients (42.4%) switched to an alternative TKI, 28 for intolerance (26.4%) and 17 (16%) for resistance. Most patients who remained on 1L-2GTKI achieved deep molecular responses (DMR) and 15 (14.1%) are in treatment-free remission (TFR). Intolerant patients also obtained DMR, although most required multiple TKI changes and were slower to respond, particularly if treated with 2L-imatinib. Inferior outcomes were observed in resistant patients, who failed alternative 2L-2GTKI and required 3/4GTKI and/or allogeneic hematopoietic stem cell transplant (alloSCT). 7yr-OS was significantly lower for these individuals (66.1%) than for intolerant patients and those who remained on 1L-2GTKI (100% and 97.9%, respectively; p = 0.001). It is apparent that failure of 1L-2GTKI is a challenging problem in modern CML therapy. Intolerance can be effectively managed by switching to an alternative 2GTKI, but resistance requires early consideration of 3/4GTKI.
© 2024. The Author(s).
Conflict of interest statement
S.C., F.C., A.K., C.H., F.F., J.K., V.O., G.S., A.I.: none. J.F.A.: advisory boards and speaker for Incyte and Novartis, research funding from Incyte and Pfizer. D.M.: honoraria from Incyte, Novartis and Pfizer; research funding from Incyte and Pfizer.
Figures
References
-
- Kantarjian HM, Hughes TP, Larson RA, Kim DW, Issaragrisil S, le Coutre P, et al. Long-term outcomes with frontline nilotinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase: ENESTnd 10-year analysis. Leukemia. 2021;35:440–53. doi: 10.1038/s41375-020-01111-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

