In situ analysis of osmolyte mechanisms of proteome thermal stabilization
- PMID: 38424171
- PMCID: PMC11288892
- DOI: 10.1038/s41589-024-01568-7
In situ analysis of osmolyte mechanisms of proteome thermal stabilization
Abstract
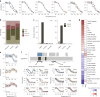
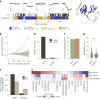
Organisms use organic molecules called osmolytes to adapt to environmental conditions. In vitro studies indicate that osmolytes thermally stabilize proteins, but mechanisms are controversial, and systematic studies within the cellular milieu are lacking. We analyzed Escherichia coli and human protein thermal stabilization by osmolytes in situ and across the proteome. Using structural proteomics, we probed osmolyte effects on protein thermal stability, structure and aggregation, revealing common mechanisms but also osmolyte- and protein-specific effects. All tested osmolytes (trimethylamine N-oxide, betaine, glycerol, proline, trehalose and glucose) stabilized many proteins, predominantly via a preferential exclusion mechanism, and caused an upward shift in temperatures at which most proteins aggregated. Thermal profiling of the human proteome provided evidence for intrinsic disorder in situ but also identified potential structure in predicted disordered regions. Our analysis provides mechanistic insight into osmolyte function within a complex biological matrix and sheds light on the in situ prevalence of intrinsically disordered regions.
© 2024. The Author(s).
Conflict of interest statement
P.P. is an inventor of a patent licensed by Biognosys AG that covers the LiP–MS method used in this manuscript (patent number WO-A-2014082733). P.P. is also a scientific advisor of Biognosys AG. The remaining authors declare no competing interests.
Figures




References
-
- Ye, Y. et al. Global metabolomic responses of Escherichia coli to heat stress. J. Proteome Res.11, 2559–2566 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 866004/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 51NF40_180541/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
LinkOut - more resources
Full Text Sources

