Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer
- PMID: 38424190
- PMCID: PMC10985007
- DOI: 10.1038/s12276-024-01180-8
Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer
Abstract
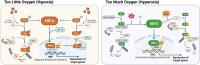
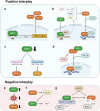
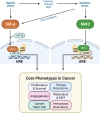
Oxygen is crucial for life and acts as the final electron acceptor in mitochondrial energy production. Cells adapt to varying oxygen levels through intricate response systems. Hypoxia-inducible factors (HIFs), including HIF-1α and HIF-2α, orchestrate the cellular hypoxic response, activating genes to increase the oxygen supply and reduce expenditure. Under conditions of excess oxygen and resulting oxidative stress, nuclear factor erythroid 2-related factor 2 (NRF2) activates hundreds of genes for oxidant removal and adaptive cell survival. Hypoxia and oxidative stress are core hallmarks of solid tumors and activated HIFs and NRF2 play pivotal roles in tumor growth and progression. The complex interplay between hypoxia and oxidative stress within the tumor microenvironment adds another layer of intricacy to the HIF and NRF2 signaling systems. This review aimed to elucidate the dynamic changes and functions of the HIF and NRF2 signaling pathways in response to conditions of hypoxia and oxidative stress, emphasizing their implications within the tumor milieu. Additionally, this review explored the elaborate interplay between HIFs and NRF2, providing insights into the significance of these interactions for the development of novel cancer treatment strategies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

