Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma
- PMID: 38424197
- PMCID: PMC11984461
- DOI: 10.1038/s41571-024-00868-0
Adjuvant and neoadjuvant immunotherapies in hepatocellular carcinoma
Abstract
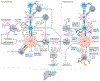
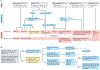
Liver cancer, specifically hepatocellular carcinoma (HCC), is the sixth most common cancer and the third leading cause of cancer mortality worldwide. The development of effective systemic therapies, particularly those involving immune-checkpoint inhibitors (ICIs), has substantially improved the outcomes of patients with advanced-stage HCC. Approximately 30% of patients are diagnosed with early stage disease and currently receive potentially curative therapies, such as resection, liver transplantation or local ablation, which result in median overall survival durations beyond 60 months. Nonetheless, up to 70% of these patients will have disease recurrence within 5 years of resection or local ablation. To date, the results of randomized clinical trials testing adjuvant therapy in patients with HCC have been negative. This major unmet need has been addressed with the IMbrave 050 trial, demonstrating a recurrence-free survival benefit in patients with a high risk of relapse after resection or local ablation who received adjuvant atezolizumab plus bevacizumab. In parallel, studies testing neoadjuvant ICIs alone or in combination in patients with early stage disease have also reported efficacy. In this Review, we provide a comprehensive overview of the current approaches to manage patients with early stage HCC. We also describe the tumour immune microenvironment and the mechanisms of action of ICIs and cancer vaccines in this setting. Finally, we summarize the available evidence from phase II/III trials of neoadjuvant and adjuvant approaches and discuss emerging clinical trials, identification of biomarkers and clinical trial design considerations for future studies.
© 2024. Springer Nature Limited.
Conflict of interest statement
J.M.L. receives research support from Bayer HealthCare Pharmaceuticals, Eisai Inc. and Sagimet; has received consulting fees from AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol–Myers Squibb, Eisai Inc., Exelixis, Genentech, Glycotest, Merck, Moderna and Roche. M.Y. has received institutional research support from Bristol–Myers Squibb, Genentech and Incyte; honoraria from Astrazeneca, Eisai, Exelixis, Genentech, Hepion and Replimune; and is a co-founder of and holds equity in Adventris Pharmaceuticals. A.G.S. has served as a consultant or on advisory boards for AstraZeneca, Bayer, Boston Scientific, Eisai, Exact Sciences, Exelixis, Freenome, FujiFilm Medical Sciences, GRAIL, Genentech, Glycotest, Roche and Universal Dx. T.U.M. has served on advisory and/or data safety monitoring boards for AbbVie, Arcus, Astellas, AstraZeneca, Atara, Boehringer Ingelheim, Bristol–Meyers Squibb, Celldex, Chimeric, DBV Technologies, DrenBio, G1 Therapeutics, Genentech, Glenmark, Merck, NGMbio, Regeneron, Rockefeller University, Simcere and Surface; and received research grants from Boehringer Ingelheim, Bristol–Myers Squibb, Merck and Regeneron. M.K. has received research support from Bayer Pharmaceutical, Chugai, Eisai, Ono Pharmaceutical and Takeda; consultancy or lecture fees from AbbVie, AstraZeneca, Bayer, Chugai, EA Pharma, Eisai, Eli Lilly, GE Healthcare, Gilead Sciences, Merck, Otsuka, Roche, Sumitomo Dainippon Pharma and Takeda. R.P., M.S., E.P. and R.S.F. declare no competing interests.
Figures
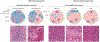

References
-
- Llovet JM et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim 7, 7 (2021). - PubMed
-
- Llovet JM et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med 359, 378–390 (2008). - PubMed
-
- Finn RS et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med 382, 1894–1905 (2020). - PubMed
-
- Llovet JM et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol 19, 151–172 (2022). - PubMed

