Hygrothermal stress increases malignant arrhythmias susceptibility by inhibiting the LKB1-AMPK-Cx43 pathway
- PMID: 38424223
- PMCID: PMC10904738
- DOI: 10.1038/s41598-024-55804-0
Hygrothermal stress increases malignant arrhythmias susceptibility by inhibiting the LKB1-AMPK-Cx43 pathway
Abstract
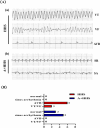
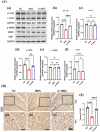
High mortality due to hygrothermal stress during heat waves is mostly linked to cardiovascular malfunction, the most serious of which are malignant arrhythmias. However, the mechanism associated with hygrothermal stress leading to malignant arrhythmias remains unclear. The energy metabolism regulated by liver kinase B1 (LKB1) and adenosine monophosphate-activated protein kinase (AMPK) and the electrical signaling based on gap junction protein, connexin43 (Cx43), plays important roles in the development of cardiac arrhythmias. In order to investigate whether hygrothermal stress induces arrhythmias via the LKB1-AMPK-Cx43 pathway, Sprague-Dawley rats were exposed to high temperature and humidity for constructing the hygrothermal stress model. A final choice of 40 °C and 85% humidity was made by pre-exploration based on different gradient environmental conditions with reference to arrhythmia event-inducing stability and risk of sudden death. Then, the incidence of arrhythmic events, as well as the expression, phosphorylation at Ser368, and distribution of Cx43 in the myocardium, were examined. Meanwhile, the adenosine monophosphate-activated protein kinase activator, Acadesine, was also administered to investigate the role played by AMPK in the process. Our results showed that hygrothermal stress induced malignant arrhythmias such as ventricular tachycardia, ventricular fibrillation, and severe atrioventricular block. Besides, hygrothermal stress decreased the phosphorylation of Cx43 at Ser368, induced proarrhythmic redistribution of Cx43 from polar to lateral sides of the cardiomyocytes, and also caused LKB1 and phosphorylated-AMPK expression to be less abundant. While, pretreatment with Acadesine significantly actived the LKB1-AMPK-Cx43 pathway and thus ameliorated malignant arrhythmias, indicating that the hygrothermal stress-induced arrhythmias is associated with the redistribution of gap junctions in cardiomyocytes and the organism's energy metabolism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
A comparison of LKB1/AMPK/mTOR metabolic axis response to global ischaemia in brain, heart, liver and kidney in a rat model of cardiac arrest.BMC Cell Biol. 2018 Jun 19;19(1):7. doi: 10.1186/s12860-018-0159-y. BMC Cell Biol. 2018. PMID: 29921218 Free PMC article.
-
Roles of 3,4-methylenedioxymethamphetamine (MDMA)-induced alteration of connexin43 and intracellular Ca(2+) oscillation in its cardiotoxicity.Toxicology. 2013 Aug 9;310:61-72. doi: 10.1016/j.tox.2013.05.013. Epub 2013 Jun 6. Toxicology. 2013. PMID: 23747752
-
PKCɛ mediates serine phosphorylation of connexin43 induced by lysophosphatidylcholine in neonatal rat cardiomyocytes.Toxicology. 2013 Dec 6;314(1):11-21. doi: 10.1016/j.tox.2013.08.001. Epub 2013 Aug 20. Toxicology. 2013. PMID: 23973256
-
Post translational modifications of connexin 43 in ventricular arrhythmias after myocardial infarction.Mol Biol Rep. 2024 Feb 23;51(1):329. doi: 10.1007/s11033-024-09290-2. Mol Biol Rep. 2024. PMID: 38393658 Review.
-
[Remodeling of cardiac gap junctions and arrhythmias].Sheng Li Xue Bao. 2011 Dec 25;63(6):586-92. Sheng Li Xue Bao. 2011. PMID: 22193455 Review. Chinese.
Cited by
-
Clinical Impact of Connexin 43 Deregulation on Myocardial Infraction.Maedica (Bucur). 2024 Jun;19(2):373-379. doi: 10.26574/maedica.2024.19.2.373. Maedica (Bucur). 2024. PMID: 39188848 Free PMC article. Review.
-
Connexin-43 Protein Expression Pattern Analysis in Myocardial Infarction Tissues.In Vivo. 2025 May-Jun;39(3):1370-1377. doi: 10.21873/invivo.13940. In Vivo. 2025. PMID: 40295004 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

