mTert induction in p21-positive cells counteracts capillary rarefaction and pulmonary emphysema
- PMID: 38424230
- PMCID: PMC10933469
- DOI: 10.1038/s44319-023-00041-1
mTert induction in p21-positive cells counteracts capillary rarefaction and pulmonary emphysema
Abstract
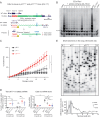
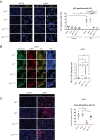
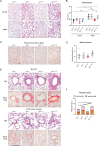
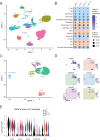
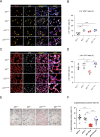
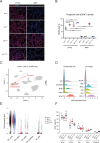
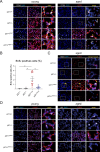
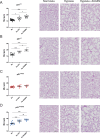
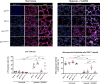
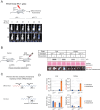
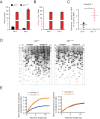
Lung diseases develop when telomeres shorten beyond a critical point. We constructed a mouse model in which the catalytic subunit of telomerase (mTert), or its catalytically inactive form (mTertCI), is expressed from the p21Cdkn1a locus. Expression of either TERT or TERTCI reduces global p21 levels in the lungs of aged mice, highlighting TERT non-canonical function. However, only TERT reduces accumulation of very short telomeres, oxidative damage, endothelial cell (ECs) senescence and senile emphysema in aged mice. Single-cell analysis of the lung reveals that p21 (and hence TERT) is expressed mainly in the capillary ECs. We report that a fraction of capillary ECs marked by CD34 and endowed with proliferative capacity declines drastically with age, and this is counteracted by TERT but not TERTCI. Consistently, only TERT counteracts decline of capillary density. Natural aging effects are confirmed using the experimental model of emphysema induced by VEGFR2 inhibition and chronic hypoxia. We conclude that catalytically active TERT prevents exhaustion of the putative CD34 + EC progenitors with age, thus protecting against capillary vessel loss and pulmonary emphysema.
Keywords: Capillary Density; Emphysema; Senescence; Telomerase; p21.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- Equipe Labellisée/Ligue Contre le Cancer (French League Against Cancer)
- Comité Paris RS21/75-24/Ligue Contre le Cancer (French League Against Cancer)
- PLBIO2019/Institut National Du Cancer (INCa)
- AGEMED/Institut National de la Santé et de la Recherche Médicale (Inserm)
- Projet Emergent/Canceropôle PACA (Canceropole PACA)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

