CAMSAPs and nucleation-promoting factors control microtubule release from γ-TuRC
- PMID: 38424271
- PMCID: PMC10940162
- DOI: 10.1038/s41556-024-01366-2
CAMSAPs and nucleation-promoting factors control microtubule release from γ-TuRC
Abstract
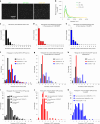
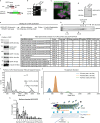
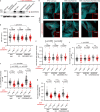
γ-Tubulin ring complex (γ-TuRC) is the major microtubule-nucleating factor. After nucleation, microtubules can be released from γ-TuRC and stabilized by other proteins, such as CAMSAPs, but the biochemical cross-talk between minus-end regulation pathways is poorly understood. Here we reconstituted this process in vitro using purified components. We found that all CAMSAPs could bind to the minus ends of γ-TuRC-attached microtubules. CAMSAP2 and CAMSAP3, which decorate and stabilize growing minus ends but not the minus-end tracking protein CAMSAP1, induced microtubule release from γ-TuRC. CDK5RAP2, a γ-TuRC-interactor, and CLASP2, a regulator of microtubule growth, strongly stimulated γ-TuRC-dependent microtubule nucleation, but only CDK5RAP2 suppressed CAMSAP binding to γ-TuRC-anchored minus ends and their release. CDK5RAP2 also improved selectivity of γ-tubulin-containing complexes for 13- rather than 14-protofilament microtubules in microtubule-capping assays. Knockout and overexpression experiments in cells showed that CDK5RAP2 inhibits the formation of CAMSAP2-bound microtubules detached from the microtubule-organizing centre. We conclude that CAMSAPs can release newly nucleated microtubules from γ-TuRC, whereas nucleation-promoting factors can differentially regulate this process.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial and non-financial interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
- 609822/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 91216006/ZonMw (Netherlands Organisation for Health Research and Development)
- X-Omics; 184.034.019/Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research)
- 31871356/National Natural Science Foundation of China (National Science Foundation of China)
- 32070705/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

