Markers of bone metabolism and overall survival in men with bone-metastatic hormone sensitive prostate cancer (HSPC): A subset analysis of SWOG S1216, a phase III trial of androgen deprivation with or without orteronel
- PMID: 38424319
- PMCID: PMC12036820
- DOI: 10.1038/s41391-024-00813-3
Markers of bone metabolism and overall survival in men with bone-metastatic hormone sensitive prostate cancer (HSPC): A subset analysis of SWOG S1216, a phase III trial of androgen deprivation with or without orteronel
Abstract
Background: Circulating biomarkers of bone metabolism are significantly associated with overall survival (OS) in men with advanced prostate cancer. In the SWOG S1216 phase III trial, we showed that elevated bone biomarkers are significantly associated with an increased risk of death in hormone-sensitive prostate cancer (HSPC) regardless of the status of bone metastases, identifying three risk groups with differential OS outcomes based on bone biomarker status. Here we report the association of bone biomarkers with OS in men with HSPC and documented skeletal metastases as part of a planned subset analysis of S1216.
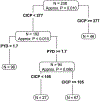
Methods: Bone resorption [C-telopeptide (CTx); Pyridinoline (PYD)] and bone formation markers [C-terminal collagen propeptide (CICP); bone alkaline phosphatase (BAP)] were assessed in blood from men with bone metastatic HSPC. Patients were randomly divided into training (n = 238) and validation (n = 475) sets. In the training set, recursive partitioning that maximizes discrimination of OS was used to identify the dichotomous cut-point for each biomarker and for a combination of biomarker split points to define prognostic groups. In the validation set, Cox proportional hazards models were used to assess the impact of biomarkers on OS, adjusted for patient and tumor characteristics.
Results: Of 1279 men, 713 had both baseline bone metastases and evaluable bone biomarkers. Patient characteristics were similar between the overall population and the subset with bone metastases. Elevated levels of CICP, CTX, and PYD were strongly prognostic for OS. Hazard ratios (95% CI) for OS adjusted for treatment arm and baseline clinical variables were: BAP-1.31 (0.93, 1.84), p = 0.12; CICP-1.58 (1.09, 2.29), p < 0.02; CTx - 1.55 (1.12, 2.15), p = 0.008; and PYD-1.66 (1.27, 2.217), p = 0.0002. There was no evidence of interaction between elevated biomarkers and treatment (all p > 0.2). Recursive partitioning algorithms identified four groups of patients with differential OS outcomes based on bone biomarkers, adjusted for baseline clinical variables, with median OS ranging from 2.3 years (highest risk group) to 7.5 years (lowest risk group).
Conclusions: In this planned S1216 subset analysis of men with HSPC and bone metastases, elevated serum markers of bone metabolism were significantly associated with worse OS. Bone biomarker levels alone and in combination with patient and tumor characteristics identify unique subsets of men with differential OS outcomes.
Gov identifier: NCT01809691.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Conflict of Interest Statement: The authors declare no potential conflicts of interest.
Figures
References
-
- Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–36. - PubMed
-
- Fortunati N, Gatti F, Felicetti F, Brignardello E. Cancer Treatment-Induced Bone Loss in Hormone-Sensitive Cancer: The Paradigm of Cancer Survivor Bone Health Management. Front Horm Res. 2021;54:91–102. - PubMed
-
- Smith MR. Markers of bone metabolism in prostate cancer. Cancer Treat Rev. 2006;32 Suppl 1:23–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P30 CA093373/CA/NCI NIH HHS/United States
- CA180819/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- CA093373/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- CA180888/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- U10 CA180888/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical