PolyGR and polyPR knock-in mice reveal a conserved neuroprotective extracellular matrix signature in C9orf72 ALS/FTD neurons
- PMID: 38424324
- PMCID: PMC11001582
- DOI: 10.1038/s41593-024-01589-4
PolyGR and polyPR knock-in mice reveal a conserved neuroprotective extracellular matrix signature in C9orf72 ALS/FTD neurons
Abstract
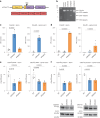
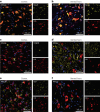
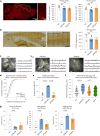
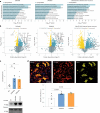
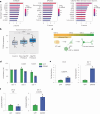
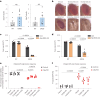
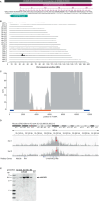
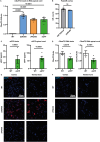
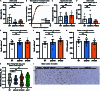
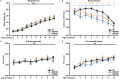
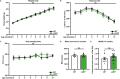
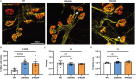
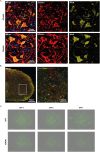
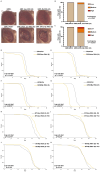
Dipeptide repeat proteins are a major pathogenic feature of C9orf72 amyotrophic lateral sclerosis (C9ALS)/frontotemporal dementia (FTD) pathology, but their physiological impact has yet to be fully determined. Here we generated C9orf72 dipeptide repeat knock-in mouse models characterized by expression of 400 codon-optimized polyGR or polyPR repeats, and heterozygous C9orf72 reduction. (GR)400 and (PR)400 knock-in mice recapitulate key features of C9ALS/FTD, including cortical neuronal hyperexcitability, age-dependent spinal motor neuron loss and progressive motor dysfunction. Quantitative proteomics revealed an increase in extracellular matrix (ECM) proteins in (GR)400 and (PR)400 spinal cord, with the collagen COL6A1 the most increased protein. TGF-β1 was one of the top predicted regulators of this ECM signature and polyGR expression in human induced pluripotent stem cell neurons was sufficient to induce TGF-β1 followed by COL6A1. Knockdown of TGF-β1 or COL6A1 orthologues in polyGR model Drosophila exacerbated neurodegeneration, while expression of TGF-β1 or COL6A1 in induced pluripotent stem cell-derived motor neurons of patients with C9ALS/FTD protected against glutamate-induced cell death. Altogether, our findings reveal a neuroprotective and conserved ECM signature in C9ALS/FTD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

